Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model
- PMID: 22448259
- PMCID: PMC3308985
- DOI: 10.1371/journal.pone.0033633
Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model
Abstract
Background: Recent understanding on cancer therapy indicated that targeting metastatic signature or angiogenic switch could be a promising and rational approach to combat cancer. Advancement in cancer research has demonstrated the potential role of various tumor suppressor proteins in inhibition of cancer progression. Current studies have shown that axonal sprouting inhibitor, semaphorin 3A (Sema 3A) acts as a potent suppressor of tumor angiogenesis in various cancer models. However, the function of Sema 3A in regulation of melanoma progression is not well studied, and yet to be the subject of intense investigation.
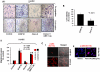
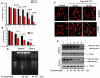
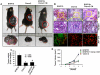
Methodology/principal findings: In this study, using multiple in vitro and in vivo approaches we have demonstrated that Sema 3A acts as a potent tumor suppressor in vitro and in vivo mice (C57BL/6) models. Mouse melanoma (B16F10) cells overexpressed with Sema 3A resulted in significant inhibition of cell motility, invasiveness and proliferation as well as suppression of in vivo tumor growth, angiogenesis and metastasis in mice models. Moreover, we have observed that Sema 3A overexpressed melanoma clone showed increased sensitivity towards curcumin and Dacarbazine, anti-cancer agents.
Conclusions: Our results demonstrate, at least in part, the functional approach underlying Sema 3A mediated inhibition of tumorigenesis and angiogenesis and a clear understanding of such a process may facilitate the development of novel therapeutic strategy for the treatment of cancer.
Conflict of interest statement
Figures




Similar articles
-
Semaphorin 3A upregulates FOXO 3a-dependent MelCAM expression leading to attenuation of breast tumor growth and angiogenesis.Oncogene. 2015 Mar 19;34(12):1584-95. doi: 10.1038/onc.2014.79. Epub 2014 Apr 14. Oncogene. 2015. PMID: 24727891
-
Direct endothelial junction restoration results in significant tumor vascular normalization and metastasis inhibition in mice.Oncotarget. 2014 May 15;5(9):2761-77. doi: 10.18632/oncotarget.1942. Oncotarget. 2014. PMID: 24811731 Free PMC article.
-
Opposite angiogenic outcome of curcumin against ischemia and Lewis lung cancer models: in silico, in vitro and in vivo studies.Biochim Biophys Acta. 2014 Sep;1842(9):1742-54. doi: 10.1016/j.bbadis.2014.06.019. Epub 2014 Jun 23. Biochim Biophys Acta. 2014. PMID: 24970744
-
Semaphorin-3A: a promising therapeutic tool in allergic rhinitis.Immunol Res. 2022 Apr;70(2):135-142. doi: 10.1007/s12026-022-09264-1. Epub 2022 Jan 14. Immunol Res. 2022. PMID: 35031951 Review.
-
Role of Semaphorin 3A in common psychiatric illnesses such as schizophrenia, depression, and anxiety.Biochem Pharmacol. 2024 Aug;226:116358. doi: 10.1016/j.bcp.2024.116358. Epub 2024 Jun 8. Biochem Pharmacol. 2024. PMID: 38857830 Review.
Cited by
-
Translational medicine: Double protection for weakened bones.Nature. 2012 May 2;485(7396):47-8. doi: 10.1038/485047a. Nature. 2012. PMID: 22552091 No abstract available.
-
Semaphorin 5A drives melanoma progression: role of Bcl-2, miR-204 and c-Myb.J Exp Clin Cancer Res. 2018 Nov 19;37(1):278. doi: 10.1186/s13046-018-0933-x. J Exp Clin Cancer Res. 2018. PMID: 30454024 Free PMC article.
-
Overexpression of Semaphorin-3A and Semaphorin-4D in the Peripheral Blood from Newly Diagnosed Patients with Chronic Lymphocytic Leukemia.Int J Hematol Oncol Stem Cell Res. 2019 Jan 1;13(1):25-34. Int J Hematol Oncol Stem Cell Res. 2019. PMID: 31205625 Free PMC article.
-
Semaphorins and plexins as therapeutic targets.Nat Rev Drug Discov. 2014 Aug;13(8):603-21. doi: 10.1038/nrd4337. Nat Rev Drug Discov. 2014. PMID: 25082288 Review.
-
Semaphorin 3A promotes osteogenic differentiation in human alveolar bone marrow mesenchymal stem cells.Exp Ther Med. 2018 Apr;15(4):3489-3494. doi: 10.3892/etm.2018.5813. Epub 2018 Jan 30. Exp Ther Med. 2018. PMID: 29545873 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. - PubMed
-
- Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–383. - PubMed
-
- Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

