Multiple Tyrosine Residues Contribute to GABA Binding in the GABA(C) Receptor Binding Pocket
- PMID: 22448304
- PMCID: PMC3309607
- DOI: 10.1021/cn200103n
Multiple Tyrosine Residues Contribute to GABA Binding in the GABA(C) Receptor Binding Pocket
Abstract
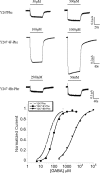
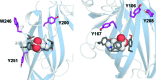
The ligand binding site of Cys-loop receptors is dominated by aromatic amino acids. In GABA(C) receptors, these are predominantly tyrosine residues, with a number of other aromatic residues located in or close to the binding pocket. Here we examine the roles of these residues using substitution with both natural and unnatural amino acids followed by functional characterization. Tyr198 (loop B) has previously been shown to form a cation-π interaction with GABA; the current data indicate that none of the other aromatic residues form such an interaction, although the data indicate that both Tyr102 and Phe138 may contribute to stabilization of the positively charged amine of GABA. Tyr247 (loop C) was very sensitive to substitution and, combined with data from a model of the receptor, suggest a π-π interaction with Tyr241 (loop C); here again functional data show aromaticity is important. In addition the hydroxyl group of Tyr241 is important, supporting the presence of a hydrogen bond with Arg104 suggested by the model. At position Tyr102 (loop D) size and aromaticity are important; this residue may play a role in receptor gating and/or ligand binding. The data also suggest that Tyr167, Tyr200, and Tyr208 have a structural role while Tyr106, Trp246, and Tyr251 are not critical. Comparison of the agonist binding site "aromatic box" across the superfamily of Cys-loop receptors reveals some interesting parallels and divergences.
Figures


Similar articles
-
Two amino acid residues contribute to a cation-π binding interaction in the binding site of an insect GABA receptor.J Neurosci. 2011 Aug 24;31(34):12371-6. doi: 10.1523/JNEUROSCI.1610-11.2011. J Neurosci. 2011. PMID: 21865479 Free PMC article.
-
Unnatural amino acid mutagenesis of the GABA(A) receptor binding site residues reveals a novel cation-pi interaction between GABA and beta 2Tyr97.J Neurosci. 2007 Jan 24;27(4):886-92. doi: 10.1523/JNEUROSCI.4791-06.2007. J Neurosci. 2007. PMID: 17251430 Free PMC article.
-
A cation-pi binding interaction with a tyrosine in the binding site of the GABAC receptor.Chem Biol. 2005 Sep;12(9):993-7. doi: 10.1016/j.chembiol.2005.06.012. Chem Biol. 2005. PMID: 16183023
-
Structural insights into Cys-loop receptor function and ligand recognition.Biochem Pharmacol. 2013 Oct 15;86(8):1042-53. doi: 10.1016/j.bcp.2013.07.001. Epub 2013 Jul 10. Biochem Pharmacol. 2013. PMID: 23850718 Review.
-
Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors.Neurochem Int. 2000 Jun;36(7):595-645. doi: 10.1016/s0197-0186(99)00154-0. Neurochem Int. 2000. PMID: 10771117 Review.
Cited by
-
GABA-ρ receptors: distinctive functions and molecular pharmacology.Br J Pharmacol. 2017 Jul;174(13):1881-1894. doi: 10.1111/bph.13768. Epub 2017 Apr 12. Br J Pharmacol. 2017. PMID: 28258627 Free PMC article. Review.
-
Molecular basis for convergent evolution of glutamate recognition by pentameric ligand-gated ion channels.Sci Rep. 2015 Feb 24;5:8558. doi: 10.1038/srep08558. Sci Rep. 2015. PMID: 25708000 Free PMC article.
-
A hydrophobic area of the GABA ρ₁ receptor containing phenylalanine 124 influences both receptor activation and deactivation.J Mol Neurosci. 2015 Feb;55(2):305-13. doi: 10.1007/s12031-014-0322-7. Epub 2014 May 10. J Mol Neurosci. 2015. PMID: 24816654
-
Molecular determinants of agonist selectivity in glutamate-gated chloride channels which likely explain the agonist selectivity of the vertebrate glycine and GABAA-ρ receptors.PLoS One. 2014 Sep 26;9(9):e108458. doi: 10.1371/journal.pone.0108458. eCollection 2014. PLoS One. 2014. PMID: 25259865 Free PMC article.
-
Identification of significant amino acids in multiple transmembrane domains of human transient receptor potential ankyrin 1 (TRPA1) for activation by eudesmol, an oxygenized sesquiterpene in hop essential oil.J Biol Chem. 2015 Jan 30;290(5):3161-71. doi: 10.1074/jbc.M114.600932. Epub 2014 Dec 18. J Biol Chem. 2015. PMID: 25525269 Free PMC article.
References
-
- Brejc K.; van Dijk W. J.; Klaassen R. V.; Schuurmans M.; van Der Oost J.; Smit A. B.; Sixma T. K. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276. - PubMed
-
- Dellisanti C. D.; Yao Y.; Stroud J. C.; Wang Z. Z.; Chen L. (2007) Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat. Neurosci. 10, 953–962. - PubMed
-
- Hilf R. J.; Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379. - PubMed
-
- Hilf R. J.; Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118. - PubMed
-
- Bocquet N.; Nury H.; Baaden M.; Le Poupon C.; Changeux J. P.; Delarue M.; Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

