EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib
- PMID: 22448344
- PMCID: PMC3308191
- DOI: 10.1158/2159-8290.CD-11-0341
EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib
Abstract
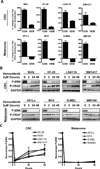
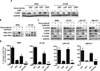
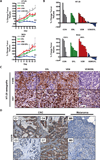
BRAF mutations occur in 10-15% of colorectal cancers (CRCs) and confer adverse outcome. While RAF inhibitors such as vemurafenib (PLX4032) have proven effective in BRAF mutant melanoma, they are surprisingly ineffective in BRAF mutant CRCs, and the reason for this disparity remains unclear. Compared to BRAF mutant melanoma cells, BRAF mutant CRC cells were less sensitive to vemurafenib, and P-ERK suppression was not sustained in response to treatment. Although transient inhibition of phospho-ERK by vemurafenib was observed in CRC, rapid ERK re-activation occurred through EGFR-mediated activation of RAS and CRAF. BRAF mutant CRCs expressed higher levels of phospho-EGFR than BRAF mutant melanomas, suggesting that CRCs are specifically poised for EGFR-mediated resistance. Combined RAF and EGFR inhibition blocked reactivation of MAPK signaling in BRAF mutant CRC cells and markedly improved efficacy in vitro and in vivo. These findings support evaluation of combined RAF and EGFR inhibition in BRAF mutant CRC patients.
Significance: BRAF valine 600 (V600) mutations occur in 10% to 15% of colorectal cancers, yet these tumors show a surprisingly low clinical response rate (~5%) to selective RAF inhibitors such as vemurafenib, which have produced dramatic response rates (60%–80%) in melanomas harboring the identical BRAF V600 mutation. We found that EGFR-mediated MAPK pathway reactivation leads to resistance to vemurafenib in BRAF-mutant colorectal cancers and that combined RAF and EGFR inhibition can lead to sustained MAPK pathway suppression and improved efficacy in vitro and in tumor xenografts.
Keywords: BRAF; EGFR; colorectal cancer; melanoma; vemurafenib.
©2012 AACR.
Figures

References
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. - PubMed
-
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. - PubMed
-
- Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. - PubMed
-
- Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

