Bortezomib salvage followed by a Phase I/II study of bortezomib plus high-dose melphalan and tandem autologous transplantation for patients with primary resistant myeloma
- PMID: 22449149
- PMCID: PMC4030553
- DOI: 10.1111/j.1365-2141.2012.09099.x
Bortezomib salvage followed by a Phase I/II study of bortezomib plus high-dose melphalan and tandem autologous transplantation for patients with primary resistant myeloma
Abstract
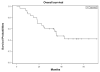
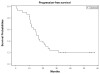
We conducted a Phase 1/2 study of bortezomib administered in combination with high-dose melphalan followed by tandem autologous transplants in patients with primary resistant multiple myeloma. Thirty patients received two cycles of salvage bortezomib followed by stem cell mobilization with granulocyte colony-stimulating factor and harvest. Melphalan 100 mg/m(2) per day on two consecutive days was administered, immediately followed by one dose of bortezomib (dose escalation) and stem cell infusion. The median beta 2-microglobulin was 4·35 mg/l (range: 1·8-11·4); albumin was 37 g/l (range: 3·1-4·9); high-risk karyotypes were noted in 45% of patients. The maximum planned dose of bortezomib at 1·3 mg/m(2) was well tolerated and a formal maximum tolerated dose was not determined. The peak of best overall response (≥partial response) and complete response rates after tandem transplants were 84% and 36%, respectively. With a median follow-up of 48 months, the median progression-free survival was 15 [95% confidence interval (CI): 11-21] months and the median overall survival was 35 (95% CI: 22-43) months. Correlative studies demonstrated decreased expression of BRCA2 (P = 0·0072) and FANCF (P = 0·0458) mRNA following bortezomib treatment. Bortezomib combined with high-dose melphalan is a well-tolerated conditioning with some activity in patients with resistant myeloma.
© 2012 Blackwell Publishing Ltd.
Figures







References
-
- Alexanian R, Dimopoulos M. The treatment of multiple myeloma. N Engl J Med. 1994;330:484–489. - PubMed
-
- Alexanian R, Weber D, Delasalle K, Handy B, Champlin R, Giralt S. Clinical outcomes with intensive therapy for patients with primary resistant multiple myeloma. Bone Marrow Transplant. 2004;34:229–234. - PubMed
-
- Andela VB, Schwarz EM, Puzas JE, O’Keefe RJ, Rosier RN. Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Res. 2000;60:6557–6562. - PubMed
-
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. - PubMed
-
- Berenson J, Yang H, Sadler K, Jarutirasarn S, Vescio R, Mapes R, Purner M, Lee S, Wilson J, Morrison B, Adams J, Schenkein D, Swift R. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24:937–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

