Immunogenicity and clinical protection against equine influenza by DNA vaccination of ponies
- PMID: 22449425
- PMCID: PMC3474358
- DOI: 10.1016/j.vaccine.2012.03.026
Immunogenicity and clinical protection against equine influenza by DNA vaccination of ponies
Abstract
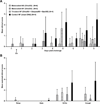
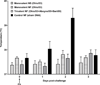
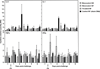
Equine influenza A (H3N8) virus infection is a leading cause of respiratory disease in horses, resulting in widespread morbidity and economic losses. As with influenza in other species, equine influenza strains continuously mutate, often requiring the development of new vaccines. Current inactivated (killed) vaccines, while efficacious, only offer limited protection against diverse subtypes and require frequent boosts. Research into new vaccine technologies, including gene-based vaccines, aims to increase the neutralization potency, breadth, and duration of protective immunity. Here, we demonstrate that a DNA vaccine expressing the hemagglutinin protein of equine H3N8 influenza virus generates homologous and heterologous immune responses, and protects against clinical disease and viral replication by homologous H3N8 virus in horses. Furthermore, we demonstrate that needle-free delivery is as efficient and effective as conventional parenteral injection using a needle and syringe. These findings suggest that DNA vaccines offer a safe, effective, and promising alternative approach for veterinary vaccines against equine influenza.
Published by Elsevier Ltd.
Conflict of interest statement
To fully disclose any potential conflicts of interest, authors Gary J. Nabel, Srinivas S. Rao, Wing-Pui Kong, and Chih-Jen Wei are each listed on a patent filing for our DNA vaccine technology, entitled “U.S. Continuation in Part Patent Application No. 12/838,292”, which is an adjunct to an existing patent entitled “Influenza DNA Vaccination and Methods of Use thereof”, Serial #61/023,341.
Figures



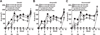
References
-
- Aerts J, Gonzalez M, Topalian S. Selection of appropriate control genes to assess expression of tumor antigens using real-time RT-PCR. Biotechniques. 2004;36:84–86. 88,90. - PubMed
-
- Amorij JP, Hinrichs WL, Frijlink HW, Wilschut JC, Huckriede A. Needle-free influenza vaccination. Lancet Infect. Dis. 2010;10:699–711. - PubMed
-
- Barouch DH, Yang Zy, Kong Wp, Korioth-Schmitz B, Sumida SM, Truitt DM, Kishko MG, Arthur JC, Miura A, Mascola JR, Letvin NL, Nabel GJ. A Human T-Cell Leukemia Virus Type 1 Regulatory Element Enhances the Immunogenicity of Human Immunodeficiency Virus Type 1 DNA Vaccines in Mice and Nonhuman Primates. J. Viral. 2005;79:8828–8834. - PMC - PubMed
-
- Boliar S, Stanislawek W, Chambers TM. Inability of kaolin treatment to remove nonspecific inhibitors from equine serum for the hemagglutination inhibition test against equine H7N7 influenza virus. J Vet Diagn Invest. 2006;18:264–267. - PubMed
-
- Breathnach CC, Clark HJ, Clark RC, Olsen CW, Townsend HG, Lunn DP. Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge. Vaccine. 2006;24:1180–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

