A mouse model of Schwartz-Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking
- PMID: 22449950
- PMCID: PMC5691331
- DOI: 10.1016/j.ajpath.2012.01.035
A mouse model of Schwartz-Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking
Abstract
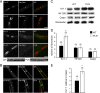
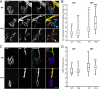
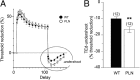
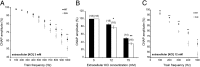
Congenital peripheral nerve hyperexcitability (PNH) is usually associated with impaired function of voltage-gated K(+) channels (VGKCs) in neuromyotonia and demyelination in peripheral neuropathies. Schwartz-Jampel syndrome (SJS) is a form of PNH that is due to hypomorphic mutations of perlecan, the major proteoglycan of basement membranes. Schwann cell basement membrane and its cell receptors are critical for the myelination and organization of the nodes of Ranvier. We therefore studied a mouse model of SJS to determine whether a role for perlecan in these functions could account for PNH when perlecan is lacking. We revealed a role for perlecan in the longitudinal elongation and organization of myelinating Schwann cells because perlecan-deficient mice had shorter internodes, more numerous Schmidt-Lanterman incisures, and increased amounts of internodal fast VGKCs. Perlecan-deficient mice did not display demyelination events along the nerve trunk but developed dysmyelination of the preterminal segment associated with denervation processes at the neuromuscular junction. Investigating the excitability properties of the peripheral nerve suggested a persistent axonal depolarization during nerve firing in vitro, most likely due to defective K(+) homeostasis, and excluded the nerve trunk as the original site for PNH. Altogether, our data shed light on perlecan function by revealing critical roles in Schwann cell physiology and suggest that PNH in SJS originates distally from synergistic actions of peripheral nerve and neuromuscular junction changes.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Hart I., Newsom-Davis J. Generalized peripheral nerve hyperexcitability (neuromyotonia) In: Engel A., Franzini-Armstrong C., editors. McGraw-Hill Medical Publishing Division; New York: 2004. pp. 1301–1309.
-
- Waxman S.G., Ritchie J.M. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. - PubMed
-
- Browne D.L., Gancher S.T., Nutt J.G., Brunt E.R., Smith E.A., Kramer P., Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. - PubMed
-
- Jen J.C., Graves T.D., Hess E.J., Hanna M.G., Griggs R.C., Baloh R.W. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain. 2007;130:2484–2493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

