A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis
- PMID: 22451060
- PMCID: PMC3690291
- DOI: 10.1002/elps.201100434
A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis
Abstract
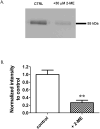
Previous studies have shown that the neurosteroid analogue, 6-Azi-pregnanolone (6-AziP), photolabels voltage-dependent anion channels and proteins of approximately 55 kDa in rat brain membranes. The present study used two-dimensional electrophoresis and nanoelectrospray ionization ion-trap mass spectrometry (nano-ESI-MS) to identify the 55 kDa proteins (isoelectric point 4.8) as isoforms of β-tubulin. This identification was confirmed by immunoblot and immunoprecipitation of photolabeled protein with anti-β-tubulin antibody and by the demonstration that 6-AziP photolabels purified bovine brain tubulin in a concentration-dependent pattern. To identify the photolabeling sites, purified bovine brain tubulin was photolabeled with 6-AziP, digested with trypsin, and analyzed by matrix-assisted laser desorption/ionization MS (MALDI). A 6-AziP adduct of TAVCDIPPR(m/z = 1287.77), a β-tubulin specific peptide, was detected by MALDI. High-resolution liquid chromatography-MS/MS analysis identified that 6-AziP was covalently bound to cysteine 354 (Cys-354), previously identified as a colchicine-binding site. 6-AziP photolabeling was inhibited by 2-methoxyestradiol, an endogenous derivative of estradiol thought to bind to the colchicine site. Structural modeling predicted that neurosteroids could dock in this colchicine site at the interface between α- and β-tubulin with the photolabeling group of 6-AziP positioned proximate to Cys-354.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
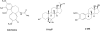



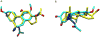
References
-
- Smith SS. Neurosteroid effects in the central nervous system: the role of the GABAA receptor. CRC Press LLC; 2004.
-
- Gasior M, Carter RB, Witkin JM. Trends Pharmacol Sci. 1999;20:107–112. - PubMed
-
- Rupprecht R. Psychoneuroendocrinology. 2003;28:139–168. - PubMed
-
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Nature. 2006;444:486–489. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

