Stratospheric ozone depletion due to nitrous oxide: influences of other gases
- PMID: 22451111
- PMCID: PMC3306630
- DOI: 10.1098/rstb.2011.0377
Stratospheric ozone depletion due to nitrous oxide: influences of other gases
Abstract
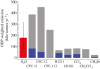
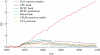
The effects of anthropogenic emissions of nitrous oxide (N(2)O), carbon dioxide (CO(2)), methane (CH(4)) and the halocarbons on stratospheric ozone (O(3)) over the twentieth and twenty-first centuries are isolated using a chemical model of the stratosphere. The future evolution of ozone will depend on each of these gases, with N(2)O and CO(2) probably playing the dominant roles as halocarbons return towards pre-industrial levels. There are nonlinear interactions between these gases that preclude unambiguously separating their effect on ozone. For example, the CH(4) increase during the twentieth century reduced the ozone losses owing to halocarbon increases, and the N(2)O chemical destruction of O(3) is buffered by CO(2) thermal effects in the middle stratosphere (by approx. 20% for the IPCC A1B/WMO A1 scenario over the time period 1900-2100). Nonetheless, N(2)O is expected to continue to be the largest anthropogenic emission of an O(3)-destroying compound in the foreseeable future. Reductions in anthropogenic N(2)O emissions provide a larger opportunity for reduction in future O(3) depletion than any of the remaining uncontrolled halocarbon emissions. It is also shown that 1980 levels of O(3) were affected by halocarbons, N(2)O, CO(2) and CH(4), and thus may not be a good choice of a benchmark of O(3) recovery.
Figures
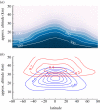
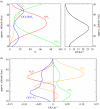
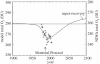
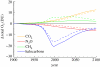

References
-
- Crutzen P. J. 1970. The influence of nitrogen oxide on the atmospheric ozone content. Q. J. R. Meteorol. Soc. 96, 320–327 10.1002/qj.49709640815 (doi:10.1002/qj.49709640815) - DOI
-
- Johnston H. S. 1971. Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust. Science 173, 517–522 10.1126/science.173.3996.517 (doi:10.1126/science.173.3996.517) - DOI - PubMed
-
- Molina M. J., Rowland F. S. 1974. Stratospheric sink for chlorofluoromethanes: chlorine atomic-catalysed destruction of ozone. Nature 249, 810–812 10.1038/249810a0 (doi:10.1038/249810a0) - DOI
-
- Stolarski R. S., Cicerone R. J. 1974. Stratospheric chlorine: possible sink for ozone. Can. J. Chem. 52, 1610–1615 10.1139/v74-233 (doi:10.1139/v74-233) - DOI
-
- Farman J., Gardiner B., Shanklin J. 1985. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 315, 207–210 10.1038/315207a0 (doi:10.1038/315207a0) - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
