Cyclin-dependent kinase 8 regulates mitotic commitment in fission yeast
- PMID: 22451489
- PMCID: PMC3372230
- DOI: 10.1128/MCB.06316-11
Cyclin-dependent kinase 8 regulates mitotic commitment in fission yeast
Erratum in
-
Correction for Szilagyi et al., "Cyclin-Dependent Kinase 8 Regulates Mitotic Commitment in Fission Yeast".Mol Cell Biol. 2017 Aug 11;37(17):e00262-17. doi: 10.1128/MCB.00262-17. Print 2017 Sep 1. Mol Cell Biol. 2017. PMID: 28801453 Free PMC article. No abstract available.
Abstract
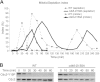
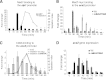
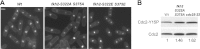
Temporal changes in transcription programs are coupled to control of cell growth and division. We here report that Mediator, a conserved coregulator of eukaryotic transcription, is part of a regulatory pathway that controls mitotic entry in fission yeast. The Mediator subunit cyclin-dependent kinase 8 (Cdk8) phosphorylates the forkhead 2 (Fkh2) protein in a periodic manner that coincides with gene activation during mitosis. Phosphorylation prevents degradation of the Fkh2 transcription factor by the proteasome, thus ensuring cell cycle-dependent variations in Fkh2 levels. Interestingly, Cdk8-dependent phosphorylation of Fkh2 controls mitotic entry, and mitotic entry is delayed by inactivation of the Cdk8 kinase activity or mutations replacing the phosphorylated serine residues of Fkh2. In addition, mutations in Fkh2, which mimic protein phosphorylation, lead to premature mitotic entry. Therefore, Fkh2 regulates not only the onset of mitotic transcription but also the correct timing of mitotic entry via effects on the Wee1 kinase. Our findings thus establish a new pathway linking the Mediator complex to control of mitotic transcription and regulation of mitotic entry in fission yeast.
Figures





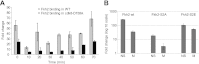
References
-
- Agarwal M, et al. 2010. Mid1p-dependent regulation of the M-G1 transcription wave in fission yeast. J. Cell Sci. 123:4366–4373 - PubMed
-
- Almonacid M, Paoletti A. 2010. Mechanisms controlling division-plane positioning. Semin. Cell Dev. Biol. 21:874–880 - PubMed
-
- Bähler J. 2005. Cell-cycle control of gene expression in budding and fission yeast. Annu. Rev. Genet. 39:69–94 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
