Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST
- PMID: 22451534
- PMCID: PMC3369700
- DOI: 10.2214/AJR.11.7483
Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST
Abstract
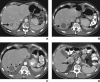
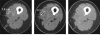
Objective: The purpose of this article is to review cancer- and therapy-specific tumor response assessment criteria used in clinical trials and in practice, with illustrative case examples, and to discuss future directions toward "personalized" tumor response assessment.
Conclusion: Although Response Evaluation Criteria in Solid Tumors will remain as the primary generalized criteria for response assessment, newer cancer- and therapy-specific criteria will play an important role in providing state-of-the-art response assessment of tumor following molecular targeted therapy and will contribute to personalized cancer care in the era of molecular medicine.
Figures



References
-
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. - PubMed
-
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
-
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

