Phosphorylation-regulated axonal dependent transport of syntaxin 1 is mediated by a Kinesin-1 adapter
- PMID: 22451907
- PMCID: PMC3326461
- DOI: 10.1073/pnas.1113819109
Phosphorylation-regulated axonal dependent transport of syntaxin 1 is mediated by a Kinesin-1 adapter
Abstract
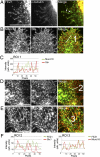
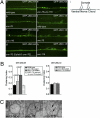
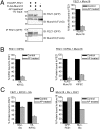
Presynaptic nerve terminals are formed from preassembled vesicles that are delivered to the prospective synapse by kinesin-mediated axonal transport. However, precisely how the various cargoes are linked to the motor proteins remains unclear. Here, we report a transport complex linking syntaxin 1a (Stx) and Munc18, two proteins functioning in synaptic vesicle exocytosis at the presynaptic plasma membrane, to the motor protein Kinesin-1 via the kinesin adaptor FEZ1. Mutation of the FEZ1 ortholog UNC-76 in Caenorhabditis elegans causes defects in the axonal transport of Stx. We also show that binding of FEZ1 to Kinesin-1 and Munc18 is regulated by phosphorylation, with a conserved site (serine 58) being essential for binding. When expressed in C. elegans, wild-type but not phosphorylation-deficient FEZ1 (S58A) restored axonal transport of Stx. We conclude that FEZ1 operates as a kinesin adaptor for the transport of Stx, with cargo loading and unloading being regulated by protein kinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

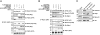

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

