Dichotomous effects of VEGF-A on adipose tissue dysfunction
- PMID: 22451920
- PMCID: PMC3326476
- DOI: 10.1073/pnas.1200447109
Dichotomous effects of VEGF-A on adipose tissue dysfunction
Abstract
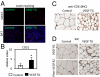
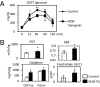
Obese fat pads are frequently undervascularized and hypoxic, leading to increased fibrosis, inflammation, and ultimately insulin resistance. We hypothesized that VEGF-A-induced stimulation of angiogenesis enables sustained and sufficient oxygen and nutrient exchange during fat mass expansion, thereby improving adipose tissue function. Using a doxycycline (Dox)-inducible adipocyte-specific VEGF-A overexpression model, we demonstrate that the local up-regulation of VEGF-A in adipocytes improves vascularization and causes a "browning" of white adipose tissue (AT), with massive up-regulation of UCP1 and PGC1α. This is associated with an increase in energy expenditure and resistance to high fat diet-mediated metabolic insults. Similarly, inhibition of VEGF-A-induced activation of VEGFR2 during the early phase of high fat diet-induced weight gain, causes aggravated systemic insulin resistance. However, the same VEGF-A-VEGFR2 blockade in ob/ob mice leads to a reduced body-weight gain, an improvement in insulin sensitivity, a decrease in inflammatory factors, and increased incidence of adipocyte death. The consequences of modulation of angiogenic activity are therefore context dependent. Proangiogenic activity during adipose tissue expansion is beneficial, associated with potent protective effects on metabolism, whereas antiangiogenic action in the context of preexisting adipose tissue dysfunction leads to improvements in metabolism, an effect likely mediated by the ablation of dysfunctional proinflammatory adipocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. - PubMed
-
- Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. - PubMed
-
- Zhang QX, et al. Vascular endothelial growth factor is the major angiogenic factor in omentum: Mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

