Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin
- PMID: 22451922
- PMCID: PMC3326483
- DOI: 10.1073/pnas.1120269109
Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin
Abstract
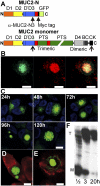
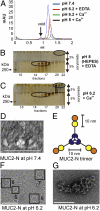
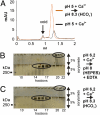
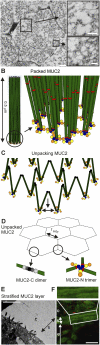
MUC2, the major colonic mucin, forms large polymers by N-terminal trimerization and C-terminal dimerization. Although the assembly process for MUC2 is established, it is not known how MUC2 is packed in the regulated secretory granulae of the goblet cell. When the N-terminal VWD1-D2-D'D3 domains (MUC2-N) were expressed in a goblet-like cell line, the protein was stored together with full-length MUC2. By mimicking the pH and calcium conditions of the secretory pathway we analyzed purified MUC2-N by gel filtration, density gradient centrifugation, and transmission electron microscopy. At pH 7.4 the MUC2-N trimer eluted as a single peak by gel filtration. At pH 6.2 with Ca(2+) it formed large aggregates that did not enter the gel filtration column but were made visible after density gradient centrifugation. Electron microscopy studies revealed that the aggregates were composed of rings also observed in secretory granulae of colon tissue sections. The MUC2-N aggregates were dissolved by removing Ca(2+) and raising pH. After release from goblet cells, the unfolded full-length MUC2 formed stratified layers. These findings suggest a model for mucin packing in the granulae and the mechanism for mucin release, unfolding, and expansion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Mucus supramolecular topology: an elusive riddle.Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):E2956; author reply E2957. doi: 10.1073/pnas.1211117109. Epub 2012 Sep 17. Proc Natl Acad Sci U S A. 2012. PMID: 22988128 Free PMC article. No abstract available.
References
-
- Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. - PubMed
-
- Gum JR, Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

