Bifunctional compounds for controlling metal-mediated aggregation of the aβ42 peptide
- PMID: 22452395
- PMCID: PMC3368506
- DOI: 10.1021/ja210588m
Bifunctional compounds for controlling metal-mediated aggregation of the aβ42 peptide
Abstract
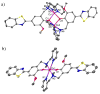
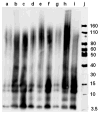
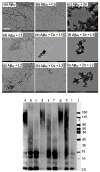
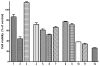
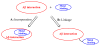
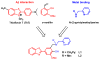
Abnormal interactions of Cu and Zn ions with the amyloid β (Aβ) peptide are proposed to play an important role in the pathogenesis of Alzheimer's disease (AD). Disruption of these metal-peptide interactions using chemical agents holds considerable promise as a therapeutic strategy to combat this incurable disease. Reported herein are two bifunctional compounds (BFCs) L1 and L2 that contain both amyloid-binding and metal-chelating molecular motifs. Both L1 and L2 exhibit high stability constants for Cu(2+) and Zn(2+) and thus are good chelators for these metal ions. In addition, L1 and L2 show strong affinity toward Aβ species. Both compounds are efficient inhibitors of the metal-mediated aggregation of the Aβ(42) peptide and promote disaggregation of amyloid fibrils, as observed by ThT fluorescence, native gel electrophoresis/Western blotting, and transmission electron microscopy (TEM). Interestingly, the formation of soluble Aβ(42) oligomers in the presence of metal ions and BFCs leads to an increased cellular toxicity. These results suggest that for the Aβ(42) peptide-in contrast to the Aβ(40) peptide-the previously employed strategy of inhibiting Aβ aggregation and promoting amyloid fibril dissagregation may not be optimal for the development of potential AD therapeutics, due to formation of neurotoxic soluble Aβ(42) oligomers.
© 2012 American Chemical Society
Figures

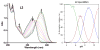
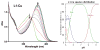
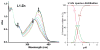
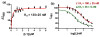



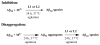
References
-
- Alzheimer’s Disease Facts and Figures Annual Report from www.alz.org.
-
- Jakob-Roetne R, Jacobsen H. Angew Chem Int Ed. 2009;48:3030. - PubMed
-
- LaFerla FM, Green KN, Oddo S. Nat Rev Neurosci. 2007;8:499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

