Myocardial β(2) -adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure
- PMID: 22452704
- PMCID: PMC3448898
- DOI: 10.1111/j.1476-5381.2012.01954.x
Myocardial β(2) -adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure
Abstract
Background and purpose: We investigated whether β(2) -adrenoceptor overexpression could promote angiogenesis and improve blood perfusion and left ventricular (LV) remodeling of the failing heart.
Experimental approach: We explored the angiogenic effects of β(2) -adrenoceptor overexpression in a rat model of post-myocardial infarction (MI) heart failure (HF). Cardiac adenoviral-mediated β(2) -adrenoceptor overexpression was obtained via direct intramyocardial injection 4-weeks post-MI. Adenovirus(Ad)-GFP and saline injected rats served as controls. Furthermore, we extended our observation to β(2) -adrenoceptor -/- mice undergoing MI.
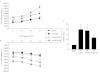
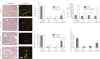
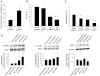
Key results: Transgenes were robustly expressed in the LV at 2 weeks post-gene therapy, whereas their expression was minimal at 4-weeks post-gene delivery. In HF rats, cardiac β(2) -adrenoceptor overexpression resulted in enhanced basal and isoprenaline-stimulated cardiac contractility at 2-weeks post-gene delivery. At 4 weeks post-gene transfer, Ad-β(2) -adrenoceptor HF rats showed improved LV remodeling and cardiac function. Importantly, β(2) -adrenoceptor overexpression was associated with a markedly increased capillary and arteriolar length density and enhanced in vivo myocardial blood flow and coronary reserve. At the molecular level, cardiac β(2) -adrenoceptor gene transfer induced the activation of the VEGF/PKB/eNOS pro-angiogenic pathway. In β(2) -adrenoceptor-/- mice, we found a ~25% reduction in cardiac capillary density compared with β(2) -adrenoceptor+/+ mice. The lack of β(2) -adrenoceptors was associated with a higher mortality rate at 30 days and LV dilatation, and a worse global cardiac contractility compared with controls.
Conclusions and implication: β(2) -Adrenoceptors play an important role in the regulation of the angiogenic response in HF. The activation of VEGF/PKB/eNOS pathway seems to be strongly involved in this mechanism.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures



Similar articles
-
Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart.Cardiovasc Res. 2008 May 1;78(2):385-94. doi: 10.1093/cvr/cvm109. Epub 2007 Dec 18. Cardiovasc Res. 2008. PMID: 18093988
-
Intracoronary adenovirus-mediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart : prospects for molecular ventricular assistance.Circulation. 2000 Feb 1;101(4):408-14. doi: 10.1161/01.cir.101.4.408. Circulation. 2000. PMID: 10653833
-
Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis.Am J Physiol Heart Circ Physiol. 2017 Aug 1;313(2):H224-H236. doi: 10.1152/ajpheart.00108.2017. Epub 2017 May 26. Am J Physiol Heart Circ Physiol. 2017. PMID: 28550172
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
-
Novel polymer carriers and gene constructs for treatment of myocardial ischemia and infarction.J Control Release. 2008 Dec 18;132(3):260-6. doi: 10.1016/j.jconrel.2008.06.024. Epub 2008 Jul 6. J Control Release. 2008. PMID: 18662730 Free PMC article. Review.
Cited by
-
β Adrenergic Receptor Kinase C-Terminal Peptide Gene-Therapy Improves β2-Adrenergic Receptor-Dependent Neoangiogenesis after Hindlimb Ischemia.J Pharmacol Exp Ther. 2016 Feb;356(2):503-13. doi: 10.1124/jpet.115.228411. Epub 2015 Nov 24. J Pharmacol Exp Ther. 2016. PMID: 26604244 Free PMC article.
-
Nanomedicine for Gene Delivery for the Treatment of Cardiovascular Diseases.Curr Gene Ther. 2019;19(1):20-30. doi: 10.2174/1566523218666181003125308. Curr Gene Ther. 2019. PMID: 30280665 Free PMC article. Review.
-
Growth Hormone Deficiency Is Associated with Worse Cardiac Function, Physical Performance, and Outcome in Chronic Heart Failure: Insights from the T.O.S.CA. GHD Study.PLoS One. 2017 Jan 17;12(1):e0170058. doi: 10.1371/journal.pone.0170058. eCollection 2017. PLoS One. 2017. PMID: 28095492 Free PMC article.
-
Neuro-hormonal effects of physical activity in the elderly.Front Physiol. 2013 Dec 20;4:378. doi: 10.3389/fphys.2013.00378. Front Physiol. 2013. PMID: 24391595 Free PMC article. Review.
-
Effects of isoprenaline on endothelial connexins and angiogenesis in a human endothelial cell culture system.Naunyn Schmiedebergs Arch Pharmacol. 2015 Jan;388(1):101-8. doi: 10.1007/s00210-014-1059-0. Epub 2014 Oct 31. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25358823
References
-
- Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. - PubMed
-
- Anversa P, Beghi C, Kikkawa Y, Olivetti G. Myocardial infarction in rats. Infarct size, myocyte hypertrophy, and capillary growth. Circ Res. 1986;58:26–37. - PubMed
-
- Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, et al. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–H2449. - PubMed
-
- Ciccarelli M, Sorriento D, Cipolletta E, Santulli G, Fusco A, Zhou RH, et al. Impaired neoangiogenesis in β2-adrenoceptor gene-deficient mice: restoration by intravascular human β2-adrenoceptor gene transfer and role of NFκB and CREB transcription factors. Br J Pharmacol. 2011;162:712–721. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

