Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient
- PMID: 22452838
- PMCID: PMC3351739
- DOI: 10.1186/1750-1172-7-18
Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient
Abstract
Background: Pontocerebellar hypoplasia (PCH) is a heterogeneous group of diseases characterized by lack of development and/or early neurodegeneration of cerebellum and brainstem. According to clinical features, seven subtypes of PCH have been described, PCH type 2 related to TSEN54 mutations being the most frequent. PCH is most often autosomal recessive though de novo anomalies in the X-linked gene CASK have recently been identified in patients, mostly females, presenting with intellectual disability, microcephaly and PCH (MICPCH).
Methods: Fourteen patients (12 females and two males; aged 16 months-14 years) presenting with PCH at neuroimaging and with clinical characteristics unsuggestive of PCH1 or PCH2 were included. The CASK gene screening was performed using Array-CGH and sequencing. Clinical and neuroradiological features were collected.
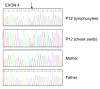
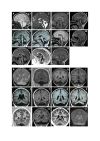
Results: We observed a high frequency of patients with a CASK mutation (13/14). Ten patients (8 girls and 2 boys) had intragenic mutations and three female patients had a Xp11.4 submicroscopic deletion including the CASK gene. All were de novo mutations. Phenotype was variable in severity but highly similar among the 11 girls and was characterized by psychomotor retardation, severe intellectual disability, progressive microcephaly, dystonia, mild dysmorphism, and scoliosis. Other signs were frequently associated, such as growth retardation, ophthalmologic anomalies (glaucoma, megalocornea and optic atrophy), deafness and epilepsy. As expected in an X-linked disease manifesting mainly in females, the boy hemizygous for a splice mutation had a very severe phenotype with nearly no development and refractory epilepsy. We described a mild phenotype in a boy with a mosaic truncating mutation. We found some degree of correlation between severity of the vermis hypoplasia and clinical phenotype.
Conclusion: This study describes a new series of PCH female patients with CASK inactivating mutations and confirms that these patients have a recognizable although variable phenotype consisting of a specific form of pontocerebellar hypoplasia. In addition, we report the second male patient to present with a severe MICPCH phenotype and a de novo CASK mutation and describe for the first time a mildly affected male patient harboring a mosaic mutation. In our reference centre, CASK related PCH is the second most frequent cause of PCH. The identification of a de novo mutation in these patients enables accurate and reassuring genetic counselling.
Figures


References
-
- Yis U, Uyanik G, Kurul S, Dirik E, Ozer E, Gross C, Hehr U. A case of Walker-Warburg syndrome resulting from a homozygous POMT1 mutation. Eur J Paediatr Neurol. 2007;11:46–49. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

