Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy
- PMID: 22452979
- PMCID: PMC3367873
- DOI: 10.1182/blood-2011-09-377846
Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy
Abstract
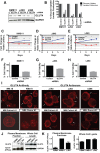
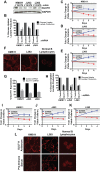
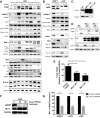
Multiple myeloma is one of numerous malignancies characterized by increased glucose consumption, a phenomenon with significant prognostic implications in this disease. Few studies have focused on elucidating the molecular underpinnings of glucose transporter (GLUT) activation in cancer, knowledge that could facilitate identification of promising therapeutic targets. To address this issue, we performed gene expression profiling studies involving myeloma cell lines and primary cells as well as normal lymphocytes to uncover deregulated GLUT family members in myeloma. Our data demonstrate that myeloma cells exhibit reliance on constitutively cell surface-localized GLUT4 for basal glucose consumption, maintenance of Mcl-1 expression, growth, and survival. We also establish that the activities of the enigmatic transporters GLUT8 and GLUT11 are required for proliferation and viability in myeloma, albeit because of functionalities probably distinct from whole-cell glucose supply. As proof of principle regarding the therapeutic potential of GLUT-targeted compounds, we include evidence of the antimyeloma effects elicited against both cell lines and primary cells by the FDA-approved HIV protease inhibitor ritonavir, which exerts a selective off-target inhibitory effect on GLUT4. Our work reveals critical roles for novel GLUT family members and highlights a therapeutic strategy entailing selective GLUT inhibition to specifically target aberrant glucose metabolism in cancer.
Figures



References
-
- Richardson PG, Schlossman R, Hideshima T, Anderson KC. New treatments for multiple myeloma. Oncology (Williston Park) 2005;19(14):1781–1792. discussion 1792,1795-1787. - PubMed
-
- Munshi N. Plasma cell disorders: an historical perspective. Hematology. 2008;1:297. - PubMed
-
- Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989–5995. - PubMed
-
- Danial NN, Gramm CF, Scorrano L, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424(6951):952–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

