Rational design and characterization of platelet factor 4 antagonists for the study of heparin-induced thrombocytopenia
- PMID: 22452981
- PMCID: PMC3383011
- DOI: 10.1182/blood-2012-01-406801
Rational design and characterization of platelet factor 4 antagonists for the study of heparin-induced thrombocytopenia
Abstract
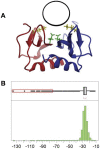
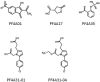
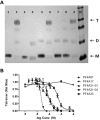
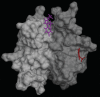
Patients with heparin-induced thrombocytopenia (HIT) remain at risk for recurrent thromboembolic complications despite improvements in management. HIT is caused by antibodies that preferentially recognize ultralarge complexes (ULCs) of heparin and platelet factor 4 (PF4) tetramers. We demonstrated previously that a variant PF4(K50E) forms dimers but does not tetramerize or form ULCs. Here, we identified small molecules predicted to bind PF4 near the dimer-dimer interface and that interfere with PF4 tetramerization. Screening a library of small molecules in silico for binding at this site, we identified 4 compounds that inhibited tetramerization at micromolar concentrations, designated PF4 antagonists (PF4As). PF4As also inhibited formation of pathogenic ULCs, and 3 of these PF4As promoted the breakdown of preformed ULCs. To characterize the ability of PF4As to inhibit cellular activation, we developed a robust and reproducible assay that measures cellular activation by HIT antibodies via FcγRIIA using DT40 cells. PF4As inhibit FcγRIIA-dependent activation of DT40 cells by HIT antibodies as well as platelet activation, as measured by serotonin release. PF4As provide new tools to probe the pathophysiology of HIT. They also may provide insight into the development of novel, disease-specific therapeutics for the treatment of thromboembolic complications in HIT.
Figures



Comment in
-
"Inactivating" PF4: a new approach to HIT treatment?Blood. 2012 Jun 21;119(25):5951-2. doi: 10.1182/blood-2012-04-422535. Blood. 2012. PMID: 22730525
References
-
- Arepally G, Cines DB. Heparin-induced thrombocytopenia and thrombosis. Clin Rev Allergy Immunol. 1998;16(3):237–247. - PubMed
-
- Warkentin TE. Heparin-induced thrombocytopenia: a ten-year retrospective. Annu Rev Med. 1999;50:129–147. - PubMed
-
- Amiral J, Bridey F, Dreyfus M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68(1):95–96. - PubMed
-
- Busch C, Dawes J, Pepper DS, Wasteson A. Binding of platelet factor 4 to cultured human umbilical vein endothelial cells. Thromb Res. 1980;19(1-2):129–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

