Dual targeting strategies with bispecific antibodies
- PMID: 22453100
- PMCID: PMC3361654
- DOI: 10.4161/mabs.4.2.19000
Dual targeting strategies with bispecific antibodies
Abstract
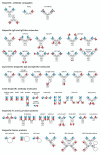
Monoclonal antibodies are widely used for the treatment of cancer, inflammatory and infectious diseases and other disorders. Most of the marketed antibodies are monospecific and therefore capable of interacting and interfering with a single target. However, complex diseases are often multifactorial in nature, and involve redundant or synergistic action of disease mediators or upregulation of different receptors, including crosstalk between their signaling networks. Consequently, blockade of multiple, different pathological factors and pathways may result in improved therapeutic efficacy. This result can be achieved by combining different drugs, or use of the dual targeting strategies applying bispecific antibodies that have emerged as an alternative to combination therapy. This review discusses the various dual targeting strategies for which bispecific antibodies have been developed and provides an overview of the established bispecific antibody formats.
Keywords: allergic diseases; bispecific antibodies; cancer therapy; dual retargeting; dual targeting; inflammatory diseases.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
