α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions
- PMID: 22453917
- PMCID: PMC3365710
- DOI: 10.1074/jbc.M111.302794
α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions
Abstract
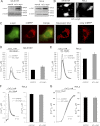
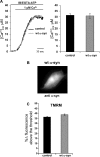
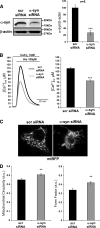
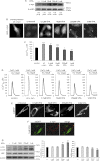
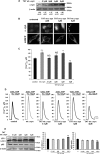
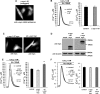
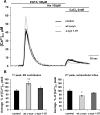
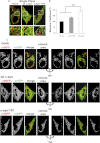
α-Synuclein has a central role in Parkinson disease, but its physiological function and the mechanism leading to neuronal degeneration remain unknown. Because recent studies have highlighted a role for α-synuclein in regulating mitochondrial morphology and autophagic clearance, we investigated the effect of α-synuclein in HeLa cells on mitochondrial signaling properties focusing on Ca(2+) homeostasis, which controls essential bioenergetic functions. By using organelle-targeted Ca(2+)-sensitive aequorin probes, we demonstrated that α-synuclein positively affects Ca(2+) transfer from the endoplasmic reticulum to the mitochondria, augmenting the mitochondrial Ca(2+) transients elicited by agonists that induce endoplasmic reticulum Ca(2+) release. This effect is not dependent on the intrinsic Ca(2+) uptake capacity of mitochondria, as measured in permeabilized cells, but correlates with an increase in the number of endoplasmic reticulum-mitochondria interactions. This action specifically requires the presence of the C-terminal α-synuclein domain. Conversely, α-synuclein siRNA silencing markedly reduces mitochondrial Ca(2+) uptake, causing profound alterations in organelle morphology. The enhanced accumulation of α-synuclein into the cells causes the redistribution of α-synuclein to localized foci and, similarly to the silencing of α-synuclein, reduces the ability of mitochondria to accumulate Ca(2+). The absence of efficient Ca(2+) transfer from endoplasmic reticulum to mitochondria results in augmented autophagy that, in the long range, could compromise cellular bioenergetics. Overall, these findings demonstrate a key role for α-synuclein in the regulation of mitochondrial homeostasis in physiological conditions. Elevated α-synuclein expression and/or eventually alteration of the aggregation properties cause the redistribution of the protein within the cell and the loss of modulation on mitochondrial function.
Figures

References
-
- Hirsch E. C., Hunot S., Faucheux B., Agid Y., Mizuno Y., Mochizuki H., Tatton W. G., Tatton N., Olanow W. C. (1999) Dopaminergic neurons degenerate by apoptosis in Parkinson disease. Mov. Disord. 14, 383–385 - PubMed
-
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 - PubMed
-
- Thomas B., Beal M. F. (2007) Parkinson disease. Hum. Mol. Genet. 16, R183–R194 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

