The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55
- PMID: 22454039
- PMCID: PMC3669693
- DOI: 10.1007/s11481-012-9351-6
The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55
Abstract
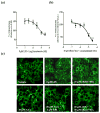
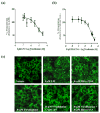
The role of cannabinoid receptors in inflammation has been the topic of many research endeavors. Despite this effort, to date the involvement of the endocannabinoid system (ECS) in inflammation remains obscure. The ambiguity of cannabinoid involvement may be explained by the existence of cannabinoid receptors, other than CB(1) and CB(2), or a consequence of interaction of endocannabinoids with other signaling systems. GPR55 has been proposed to be a cannabinoid receptor; however the interaction of the endocannabinoid system with GPR55 remains elusive. Consequently this study set about to examine the effects of the endocannabinoids, anandamide (AEA) and virodhamine, on GPR55 mediated signaling. Specifically, we assessed changes in β-arrestin2 (βarr2) distribution and GPR55 receptor internalization following activation by lysophosphatidylinositol (LPI), the synthetic cannabinoid ligand SR141716A, and new selective synthetic GPR55 agonists. Data obtained from the experiments presented herein demonstrate that AEA and virodhamine modulate agonist-mediated recruitment of βarr2. AEA and virodhamine act as partial agonists; enhancing the agonist effect at low concentrations and inhibiting it at high concentrations. Furthermore, both virodhamine and AEA significantly attenuated agonist-induced internalization of GPR55. These effects are attributed to the expression of GPR55, and not CB(1) and CB(2) receptors, as we have established negligible expression of CB(1) and CB(2) in these GPR55-transfected U2OS cells. The identification of select endocannabinoids as GPR55 modulators will aide in elucidating the function of GPR55 in the ECS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
The molecular basis for neuroimmune receptor signaling.J Neuroimmune Pharmacol. 2012 Dec;7(4):722-4. doi: 10.1007/s11481-012-9398-4. Epub 2012 Aug 31. J Neuroimmune Pharmacol. 2012. PMID: 22935971 Free PMC article.
References
-
- Abood ME, Rizvi G, Sallapudi N, McAllister SD. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neuroscience letters. 2001;309 (3):197–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

