Comparison and combination of blood-based inflammatory markers with faecal occult blood tests for non-invasive colorectal cancer screening
- PMID: 22454079
- PMCID: PMC3326680
- DOI: 10.1038/bjc.2012.104
Comparison and combination of blood-based inflammatory markers with faecal occult blood tests for non-invasive colorectal cancer screening
Abstract
Background: Faecal occult blood tests (FOBTs) are widely used for colorectal cancer (CRC) screening. Blood-based inflammatory markers have been suggested as alternative or supplementary non-invasive CRC screening tests.
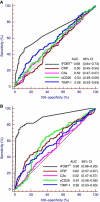
Methods: Among 179 CRC patients, 193 people with advanced adenoma and 225 people free of neoplasm, C-reactive protein (CRP), serum CD26 (sCD26), complement C3a anaphylatoxin and tissue inhibitor of metalloproteinases 1 (TIMP-1) levels in blood were measured by ELISA tests, and an immunochemical FOBT (iFOBT) and a guaiac-based FOBT were performed. Receiver operating characteristic curves were constructed and the areas under the curves (AUCs) were compared.
Results: The blood levels of CRP, sCD26 and TIMP-1 showed statistically significant differences between CRC patients and neoplasm-free participants, and levels of TIMP-1 were furthermore significantly elevated in advanced adenoma patients. For the four inflammatory markers, AUCs ranged from 0.52 to 0.62 for CRC detection and from 0.50 to 0.58 for advanced adenomas detection, compared with AUCs of 0.90 and 0.68 for the iFOBT. At 97% specificity, blood markers showed much lower sensitivities than FOBTs. Combining inflammatory markers with the iFOBT increased the AUC for advanced adenomas.
Conclusion: These blood-based markers do not seem to be an alternative to FOBT-based CRC screening. The potential use of these and other blood-based tests in combination with iFOBT might deserve further attention.
Figures

References
-
- Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M (2011) Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 154: 22–30 - PubMed
-
- Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M (2007) Case-control study supports extension of surveillance interval after colonoscopicpolypectomy to at least 5 yr. Am J Gastroenterol 102: 1739–1744 - PubMed
-
- Brenner H, Haug U, Hundt S (2010a) Inter-test agreement and quantitative cross-validation of immunochromatographicalfecal occult blood tests. Int J Cancer 127: 1643–1649 - PubMed
-
- Brenner H, Haug U, Hundt S (2010b) Sex differences in performance of fecal occult blood testing. Am J Gastroenterol 105: 2457–2464 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

