Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data
- PMID: 22454089
- PMCID: PMC3314184
- DOI: 10.1136/bmj.e1401
Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data
Abstract
Objectives: To determine the effect of human papillomavirus (HPV) quadrivalent vaccine on the risk of developing subsequent disease after an excisional procedure for cervical intraepithelial neoplasia or diagnosis of genital warts, vulvar intraepithelial neoplasia, or vaginal intraepithelial neoplasia.
Design: Retrospective analysis of data from two international, double blind, placebo controlled, randomised efficacy trials of quadrivalent HPV vaccine (protocol 013 (FUTURE I) and protocol 015 (FUTURE II)).
Setting: Primary care centres and university or hospital associated health centres in 24 countries and territories around the world.
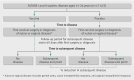
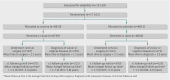
Participants: Among 17,622 women aged 15-26 years who underwent 1:1 randomisation to vaccine or placebo, 2054 received cervical surgery or were diagnosed with genital warts, vulvar intraepithelial neoplasia, or vaginal intraepithelial neoplasia.
Intervention: Three doses of quadrivalent HPV vaccine or placebo at day 1, month 2, and month 6.
Main outcome measures: Incidence of HPV related disease from 60 days after treatment or diagnosis, expressed as the number of women with an end point per 100 person years at risk.
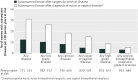
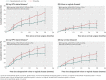
Results: A total of 587 vaccine and 763 placebo recipients underwent cervical surgery. The incidence of any subsequent HPV related disease was 6.6 and 12.2 in vaccine and placebo recipients respectively (46.2% reduction (95% confidence interval 22.5% to 63.2%) with vaccination). Vaccination was associated with a significant reduction in risk of any subsequent high grade disease of the cervix by 64.9% (20.1% to 86.3%). A total of 229 vaccine recipients and 475 placebo recipients were diagnosed with genital warts, vulvar intraepithelial neoplasia, or vaginal intraepithelial neoplasia, and the incidence of any subsequent HPV related disease was 20.1 and 31.0 in vaccine and placebo recipients respectively (35.2% reduction (13.8% to 51.8%)).
Conclusions: Previous vaccination with quadrivalent HPV vaccine among women who had surgical treatment for HPV related disease significantly reduced the incidence of subsequent HPV related disease, including high grade disease.
Trial registrations: NCT00092521 and NCT00092534.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Effect of quadrivalent HPV vaccination on HPV related disease in women treated for cervical or vulvar/vaginal disease.BMJ. 2012 Mar 27;344:e1544. doi: 10.1136/bmj.e1544. BMJ. 2012. PMID: 22454090 No abstract available.
-
Reduction in subsequent HPV related disease was unrelated to vaccination.BMJ. 2012 May 8;344:e3195; author reply e3198. doi: 10.1136/bmj.e3195. BMJ. 2012. PMID: 22570208 No abstract available.
References
-
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928-43. - PubMed
-
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915-27. - PubMed
-
- The FUTURE II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3 and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007;369:1861-8. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007;369:2161-70. - PubMed
-
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301-14. - PubMed
