Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401
- PMID: 22454414
- PMCID: PMC3383121
- DOI: 10.1200/JCO.2011.39.4767
Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401
Abstract
Purpose: A randomized, placebo-controlled study based on preclinical and clinical data that supports the potential role of vascular endothelial growth factor in prostate cancer was performed to evaluate the addition of bevacizumab to standard docetaxel and prednisone therapy in patients with metastatic castration-resistant prostate cancer (mCRPC).
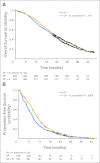
Patients and methods: Patients with chemotherapy-naive progressive mCRPC with Eastern Cooperative Oncology Group performance status ≤ 2 and adequate bone marrow, hepatic, and renal function were randomly assigned to receive docetaxel 75 mg/m(2) intravenously (IV) over 1 hour for 21 days plus prednisone 5 mg orally twice per day (DP) with either bevacizumab 15 mg/kg IV every 3 weeks (DP + B) or placebo. The primary end point was overall survival (OS), and secondary end points were progression-free survival (PFS), 50% decline in prostate-specific antigen, objective response (OR), and toxicity.
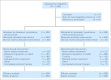
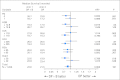
Results: In total, 1,050 patients were randomly assigned. The median OS for patients given DP + B was 22.6 months compared with 21.5 months for patients treated with DP (hazard ratio, 0.91; 95% CI, 0.78 to 1.05; stratified log-rank P = .181). The median PFS time was superior in the DP + B arm (9.9 v 7.5 months, stratified log-rank P < .001) as was the proportion of patients with OR (49.4% v 35.5%; P = .0013). Grade 3 or greater treatment-related toxicity was more common with DP + B (75.4% v 56.2%; P ≤ .001), as was the number of treatment-related deaths (4.0% v 1.2%; P = .005).
Conclusion: Despite an improvement in PFS and OR, the addition of bevacizumab to docetaxel and prednisone did not improve OS in men with mCRPC and was associated with greater toxicity.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Ferrer FA, Miller LJ, Lindquist R, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. - PubMed
-
- Ferrer FA, Miller LJ, Andrawis RI. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: In situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997;157:2329–2333. - PubMed
-
- Duque JL, Loughlin KR, Adam RM, et al. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999;54:523–527. - PubMed
-
- Duque JL, Loughlin KR, Adam RM, et al. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: Relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61:401–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

