Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma
- PMID: 22454421
- PMCID: PMC3646316
- DOI: 10.1200/JCO.2011.38.0410
Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma
Abstract
Purpose: Brentuximab vedotin is an antibody-drug conjugate (ADC) that selectively delivers monomethyl auristatin E, an antimicrotubule agent, into CD30-expressing cells. In phase I studies, brentuximab vedotin demonstrated significant activity with a favorable safety profile in patients with relapsed or refractory CD30-positive lymphomas.
Patients and methods: In this multinational, open-label, phase II study, the efficacy and safety of brentuximab vedotin were evaluated in patients with relapsed or refractory Hodgkin's lymphoma (HL) after autologous stem-cell transplantation (auto-SCT). Patients had histologically documented CD30-positive HL by central pathology review. A total of 102 patients were treated with brentuximab vedotin 1.8 mg/kg by intravenous infusion every 3 weeks. In the absence of disease progression or prohibitive toxicity, patients received a maximum of 16 cycles. The primary end point was the overall objective response rate (ORR) determined by an independent radiology review facility.
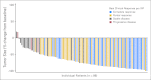
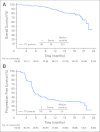
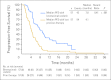
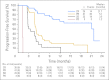
Results: The ORR was 75% with complete remission (CR) in 34% of patients. The median progression-free survival time for all patients was 5.6 months, and the median duration of response for those in CR was 20.5 months. After a median observation time of more than 1.5 years, 31 patients were alive and free of documented progressive disease. The most common treatment-related adverse events were peripheral sensory neuropathy, nausea, fatigue, neutropenia, and diarrhea.
Conclusion: The ADC brentuximab vedotin was associated with manageable toxicity and induced objective responses in 75% of patients with relapsed or refractory HL after auto-SCT. Durable CRs approaching 2 years were observed, supporting study in earlier lines of therapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Brentuximab vedotin and panobinostat: new drugs for Hodgkin's lymphoma--can they make one of medical oncology's chemotherapy success stories more successful?J Clin Oncol. 2012 Jun 20;30(18):2171-2. doi: 10.1200/JCO.2011.39.6416. Epub 2012 Apr 30. J Clin Oncol. 2012. PMID: 22547611 No abstract available.
-
Advances in LLM: Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma.Clin Adv Hematol Oncol. 2012 Jul;10(7):468-71. Clin Adv Hematol Oncol. 2012. PMID: 22895289 No abstract available.
References
-
- Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23:6400–6408. - PubMed
-
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348:2386–2395. [Erratum: N Engl J Med 353:744, 2005] - PubMed
-
- Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. - PubMed
-
- Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. - PubMed
-
- Horning S, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: An international effort. Ann Oncol. 2008;19:118. abstr.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

