Botulinum neurotoxin D-C uses synaptotagmin I and II as receptors, and human synaptotagmin II is not an effective receptor for type B, D-C and G toxins
- PMID: 22454523
- PMCID: PMC4067266
- DOI: 10.1242/jcs.103564
Botulinum neurotoxin D-C uses synaptotagmin I and II as receptors, and human synaptotagmin II is not an effective receptor for type B, D-C and G toxins
Abstract
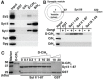
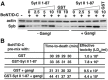
Botulinum neurotoxins (BoNTs) are classified into seven types (A-G), but multiple subtype and mosaic toxins exist. These subtype and mosaic toxins share a high sequence identity, and presumably the same receptors and substrates with their parental toxins. Here, we report that a mosaic toxin, type D-C (BoNT/D-C), uses different receptors from its parental toxin BoNT/C. BoNT/D-C, but not BoNT/C, binds directly to the luminal domains of synaptic vesicle proteins synaptotagmin (Syt) I and II, and requires expression of SytI/II to enter neurons. The SytII luminal fragment containing the toxin-binding site can block the entry of BoNT/D-C into neurons and reduce its toxicity in vivo in mice. We also found that gangliosides increase binding of BoNT/D-C to SytI/II and enhance the ability of the SytII luminal fragment to block BoNT/D-C entry into neurons. These data establish SytI/II, in conjunction with gangliosides, as the receptors for BoNT/D-C, and indicate that BoNT/D-C is functionally distinct from BoNT/C. We further found that BoNT/D-C recognizes the same binding site on SytI/II where BoNT/B and G also bind, but utilizes a receptor-binding interface that is distinct from BoNT/B and G. Finally, we also report that human and chimpanzee SytII has diminished binding and function as the receptor for BoNT/B, D-C and G owing to a single residue change from rodent SytII within the toxin binding site, potentially reducing the potency of these BoNTs in humans and chimpanzees.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

