Selective serotonergic excitation of callosal projection neurons
- PMID: 22454619
- PMCID: PMC3308333
- DOI: 10.3389/fncir.2012.00012
Selective serotonergic excitation of callosal projection neurons
Abstract
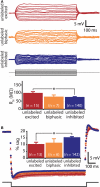
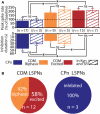
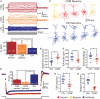
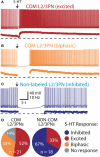
Serotonin (5-HT) acting as a neurotransmitter in the cerebral cortex is critical for cognitive function, yet how 5-HT regulates information processing in cortical circuits is not well understood. We tested the serotonergic responsiveness of layer 5 pyramidal neurons (L5PNs) in the mouse medial prefrontal cortex (mPFC), and found three distinct response types: long-lasting 5-HT(1A) (1A) receptor-dependent inhibitory responses (84% of L5PNs), 5-HT(2A) (2A) receptor-dependent excitatory responses (9%), and biphasic responses in which 2A-dependent excitation followed brief inhibition (5%). Relative to 5-HT-inhibited neurons, those excited by 5-HT had physiological properties characteristic of callosal/commissural (COM) neurons that project to the contralateral cortex. We tested whether serotonergic responses in cortical pyramidal neurons are correlated with their axonal projection pattern using retrograde fluorescent labeling of COM and corticopontine-projecting (CPn) neurons. 5-HT generated excitatory or biphasic responses in all 5-HT-responsive layer 5 COM neurons. Conversely, CPn neurons were universally inhibited by 5-HT. Serotonergic excitation of COM neurons was blocked by the 2A antagonist MDL 11939, while serotonergic inhibition of CPn neurons was blocked by the 1A antagonist WAY 100635, confirming a role for these two receptor subtypes in regulating pyramidal neuron activity. Selective serotonergic excitation of COM neurons was not layer-specific, as COM neurons in layer 2/3 were also selectively excited by 5-HT relative to their non-labeled pyramidal neuron neighbors. Because neocortical 2A receptors are implicated in the etiology and pathophysiology of schizophrenia, we propose that COM neurons may represent a novel cellular target for intervention in psychiatric disease.
Keywords: 5-HT1A receptor; 5-HT2A receptor; cerebral cortex; corpus callosum; neocortex; pyramidal neuron; retrograde labeling; serotonin.
Figures
References
-
- Amargos-Bosch M., Bortolozzi A., Puig M. V., Serrats J., Adell A., Celada P., Toth M., Mengod G., Artigas F. (2004). Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex 14, 281–299 10.1093/cercor/bhg128 - DOI - PubMed
-
- Beique J. C., Campbell B., Perring P., Hamblin M. W., Walker P., Mladenovic L., Andrade R. (2004). Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 24, 4807–4817 10.1523/JNEUROSCI.5113-03.2004 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

