Effects of Brugmansia arborea Extract and Its Secondary Metabolites on Morphine Tolerance and Dependence in Mice
- PMID: 22454681
- PMCID: PMC3290905
- DOI: 10.1155/2012/741925
Effects of Brugmansia arborea Extract and Its Secondary Metabolites on Morphine Tolerance and Dependence in Mice
Abstract
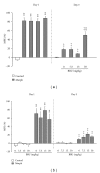
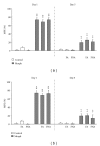
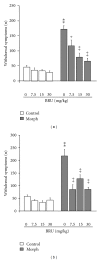
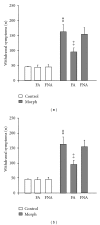
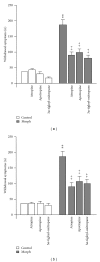
The aim of the present study was to investigate, in vivo, the effect of a Brugmansia arborea extract (BRU), chromatographic fractions (FA and FNA), and isolated alkaloids on the expression and the acquisition of morphine tolerance and dependence. Substances were acutely (for expression) or repeatedly (for acquisition) administered in mice treated with morphine twice daily for 5 or 6 days, in order to make them tolerant or dependent. Morphine tolerance was assessed using the tail-flick test at 1st and 5th days. Morphine dependence was evaluated through the manifestation of withdrawal symptoms induced by naloxone injection at 6th day. Results showed that BRU significantly reduced the expression of morphine tolerance, while it was ineffective to modulate its acquisition. Chromatographic fractions and pure alkaloids failed to reduce morphine tolerance. Conversely BRU, FA, and pure alkaloids administrations significantly attenuated both development and expression of morphine dependence. These data suggest that Brugmansia arborea Lagerh might have human therapeutic potential for treatment of opioid addiction.
Figures

References
-
- De Feo V. The ritual use of Brugmansia species in traditional andean medicine in Northern Peru. Economic Botany. 2004;58:S221–S229.
-
- Roses OE, Lopez CM, Garcia Fernandez JC. Aislamiento e identificación de alcaloides del tropano en especies del género Brugmansia (Solanaceae) Acta Farmacéutica Bonaerense. 1987;6:167–174.
-
- Capasso A, De Feo V, De Simone F, Sorrentino L. Activity-directed isolation of spasmolytic (anti-cholinergic) alkaloids from Brugmansia arborea (L.) Lagerheim. International Journal of Pharmacognosy. 1997;35(1):43–48.
-
- Capasso A, De Feo V. Central nervous system pharmacological effects of plants from northern Peruvian Andes: Valeriana adscendens, Iresine herbstii and Brugmansia arborea . Pharmaceutical Biology. 2002;40(4):274–293.
-
- Nencini C, Cavallo F, Bruni G, et al. Affinity of Iresine herbstii and Brugmansia arborea extracts on different cerebral receptors. Journal of Ethnopharmacology. 2006;105(3):352–357. - PubMed
LinkOut - more resources
Full Text Sources

