Myopia and inflammation
Abstract
The correlation between myopia and intraocular inflammation has rarely been explored. The aim of this article is to review myopic changes induced by inflammatory diseases and inflammatory diseases related to myopia, followed by a discussion on inflammatory choroidal neovascularization. Clinical cases are used to illustrate these conditions. The review does not include inflammatory conditions caused by surgical interventions employed for treatment of myopia. Uveitic conditions that can induce a myopic shift include sclero-choroidal inflammation, lens induced myopia due to steroid cataracts, juvenile idiopathic arthritis (JIA) induced myopia, and transient drug induced myopia due to sulfonamides and acetazolamide used for treatment of ocular toxoplasmosis and inflammatory cystoid macular edema, respectively. Most inflammatory conditions related to myopia are conditions involving the choriocapillaris. These include multifocal choroiditis and/or punctate inner choroiditis, multiple evanescent white dot syndrome and acute idiopathic blind spot enlargement. It can be hypothesized that fragility of the choriocapillaris due to particular anatomic changes due to myopia, together with unknown immunogenetic factors predispose myopic eyes to primary inflammatory choriocapillaropathies.
Keywords: Choriocapillaritis; Inflammation; Multifocal Choroiditis; Multiple Evanescent White Dot Syndrome; Myopia; Vogt-Koyanagi-Harada Disease.
Figures




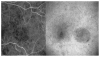





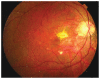
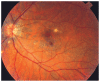
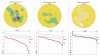
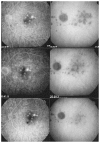
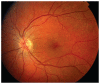

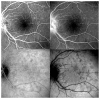

References
-
- Nomura H, Ando F, Nino N, Shimokata H, Miyake Y. The relationship between intraocular pressure and refractive error adjusting for age and central corneal thickness. Ophthalmic Physiol Opt. 2004;24:41–45. - PubMed
-
- Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility: the Beijing eye study. Ophthalmology. 2007;114:216–220. - PubMed
-
- Lee YA, Shih YF, Lin LL, Huang JY, Wang TH. Association between high myopia and progression of visual field loss in primary open-angle glaucoma. J Formos Med Assoc. 2008;107:952–957. - PubMed
-
- Bremer F. Origin of corticosteroid glaucoma. Bull Soc Belge Ophtalmol. 2007;304:111–116. - PubMed
-
- Gross SA, von Noorden GK, Jones DB. Necrotizing scleritis and transient myopia following strabismus surgery. Ophthalmic Surg. 1993;24:839–841. - PubMed
LinkOut - more resources
Full Text Sources
