Bilateral subcortical heterotopia with partial callosal agenesis in a mouse mutant
- PMID: 22455839
- PMCID: PMC3593577
- DOI: 10.1093/cercor/bhs080
Bilateral subcortical heterotopia with partial callosal agenesis in a mouse mutant
Abstract
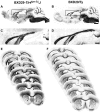
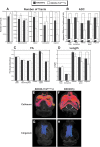
Cognition and behavior depend on the precise placement and interconnection of complex ensembles of neurons in cerebral cortex. Mutations that disrupt migration of immature neurons from the ventricular zone to the cortical plate have provided major insight into mechanisms of brain development and disease. We have discovered a new and highly penetrant spontaneous mutation that leads to large nodular bilateral subcortical heterotopias with partial callosal agenesis. The mutant phenotype was first detected in a colony of fully inbred BXD29 mice already known to harbor a mutation in Tlr4. Neurons confined to the heterotopias are mainly born in midgestation to late gestation and would normally have migrated into layers 2-4 of overlying neocortex. Callosal cross-sectional area and fiber number are reduced up to 50% compared with coisogenic wildtype BXD29 substrain controls. Mutants have a pronounced and highly selective defect in rapid auditory processing. The segregation pattern of the mutant phenotype is most consistent with a two-locus autosomal recessive model, and selective genotyping definitively rules out the Tlr4 mutation as a cause. The discovery of a novel mutation with strong pleiotropic anatomical and behavioral effects provides an important new resource for dissecting molecular mechanisms and functional consequences of errors of neuronal migration.
Figures




References
-
- Andrade DM. Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain. Hum Genet. 2009;126:173–193. - PubMed
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. - PubMed
-
- Bilasy SE, Satoh T, Ueda S, Wei P, Kanemura H, Aiba A, Terashima T, Kataoka T. Dorsal telencephalon-specific RA-GEF-1 knockout mice develop heterotopic cortical mass and commissural fiber defect. Eur J Neurosci. 2009;29:1994–2008. - PubMed
-
- Caviness VS, Jr, Frost DO. Thalamocortical projections in the reeler mutant mouse. J Comp Neurol. 1983;219:182–202. - PubMed
-
- Caviness VS, Jr, So DK, Sidman RL. The hybrid reeler mouse. J Hered. 1972;63:241–246. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

