Doing it in reverse: 3'-to-5' polymerization by the Thg1 superfamily
- PMID: 22456265
- PMCID: PMC3334698
- DOI: 10.1261/rna.032300.112
Doing it in reverse: 3'-to-5' polymerization by the Thg1 superfamily
Abstract
The tRNA(His) guanylyltransferase (Thg1) family of enzymes comprises members from all three domains of life (Eucarya, Bacteria, Archaea). Although the initial activity associated with Thg1 enzymes was a single 3'-to-5' nucleotide addition reaction that specifies tRNA(His) identity in eukaryotes, the discovery of a generalized base pair-dependent 3'-to-5' polymerase reaction greatly expanded the scope of Thg1 family-catalyzed reactions to include tRNA repair and editing activities in bacteria, archaea, and organelles. While the identification of the 3'-to-5' polymerase activity associated with Thg1 enzymes is relatively recent, the roots of this discovery and its likely physiological relevance were described ≈ 30 yr ago. Here we review recent advances toward understanding diverse Thg1 family enzyme functions and mechanisms. We also discuss possible evolutionary origins of Thg1 family-catalyzed 3'-to-5' addition activities and their implications for the currently observed phylogenetic distribution of Thg1-related enzymes in biology.
Figures

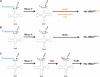




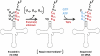
Similar articles
-
tRNAHis-guanylyltransferase establishes tRNAHis identity.Nucleic Acids Res. 2012 Jan;40(1):333-44. doi: 10.1093/nar/gkr696. Epub 2011 Sep 2. Nucleic Acids Res. 2012. PMID: 21890903 Free PMC article.
-
A role for tRNA(His) guanylyltransferase (Thg1)-like proteins from Dictyostelium discoideum in mitochondrial 5'-tRNA editing.RNA. 2011 Apr;17(4):613-23. doi: 10.1261/rna.2517111. Epub 2011 Feb 9. RNA. 2011. PMID: 21307182 Free PMC article.
-
tRNA 5'-end repair activities of tRNAHis guanylyltransferase (Thg1)-like proteins from Bacteria and Archaea.Nucleic Acids Res. 2011 Mar;39(5):1833-42. doi: 10.1093/nar/gkq976. Epub 2010 Nov 3. Nucleic Acids Res. 2011. PMID: 21051361 Free PMC article.
-
The Role of 3' to 5' Reverse RNA Polymerization in tRNA Fidelity and Repair.Genes (Basel). 2019 Mar 26;10(3):250. doi: 10.3390/genes10030250. Genes (Basel). 2019. PMID: 30917604 Free PMC article. Review.
-
This is the end: processing, editing and repair at the tRNA 3'-terminus.Biol Chem. 2001 Aug;382(8):1147-56. doi: 10.1515/BC.2001.144. Biol Chem. 2001. PMID: 11592395 Review.
Cited by
-
Structural studies of a bacterial tRNA(HIS) guanylyltransferase (Thg1)-like protein, with nucleotide in the activation and nucleotidyl transfer sites.PLoS One. 2013 Jul 3;8(7):e67465. doi: 10.1371/journal.pone.0067465. Print 2013. PLoS One. 2013. PMID: 23844012 Free PMC article.
-
A Temporal Order in 5'- and 3'- Processing of Eukaryotic tRNAHis.Int J Mol Sci. 2019 Mar 19;20(6):1384. doi: 10.3390/ijms20061384. Int J Mol Sci. 2019. PMID: 30893886 Free PMC article.
-
Chemical footprinting and kinetic assays reveal dual functions for highly conserved eukaryotic tRNAHis guanylyltransferase residues.J Biol Chem. 2019 May 31;294(22):8885-8893. doi: 10.1074/jbc.RA119.007939. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000629 Free PMC article.
-
Life without post-transcriptional addition of G-1: two alternatives for tRNAHis identity in Eukarya.RNA. 2015 Feb;21(2):243-53. doi: 10.1261/rna.048389.114. Epub 2014 Dec 12. RNA. 2015. PMID: 25505023 Free PMC article.
-
The life and times of a tRNA.RNA. 2023 Jul;29(7):898-957. doi: 10.1261/rna.079620.123. Epub 2023 Apr 13. RNA. 2023. PMID: 37055150 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
