Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes
- PMID: 22456356
- PMCID: PMC3445411
- DOI: 10.1097/PRS.0b013e3182450b47
Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes
Abstract
Background: Although fat deposition is a defining clinical characteristic of lymphedema, the cellular mechanisms that regulate this response remain unknown. The goal of this study was to determine how lymphatic fluid stasis regulates adipogenic gene activation and fat deposition.
Methods: Adult female mice underwent tail lymphatic ablation and were euthanied at 1, 3, or 6 weeks postoperatively (n = 8 per group). Samples were analyzed by immunohistochemistry and Western blot analysis. An alternative group of mice underwent axillary dissections or sham incisions, and limb tissues were harvested 3 weeks postoperatively (n = 8 per group).
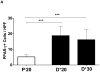
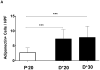
Results: Lymphatic fluid stasis resulted in significant subcutaneous fat deposition and fibrosis in lymphedematous tail regions (p < 0.001). Western blot analysis demonstrated that proteins regulating adipose differentiation including CCAAT/enhancer-binding protein-α and adiponectin were markedly up-regulated in response to lymphatic fluid stasis in the tail and axillary models. Expression of these markers increased in edematous tissues according to the gradient of lymphatic stasis distal to the wound. Immunohistochemical analysis further demonstrated that adiponectin and peroxisome proliferator-activated receptor-γ, another critical adipogenic transcription factor, followed similar expression gradients. Finally, adiponectin and peroxisome proliferator-activated receptor-γ expression localized to a variety of cell types in newly formed subcutaneous fat.
Conclusions: The mouse-tail model of lymphedema demonstrates pathologic findings similar to clinical lymphedema, including fat deposition and fibrosis. The authors show that lymphatic fluid stasis potently up-regulates the expression of fat differentiation markers both spatially and temporally. These studies elucidate mechanisms regulating abnormal fat deposition in lymphedema pathogenesis and therefore provide a basis for developing targeted treatments.
Conflict of interest statement
Figures


















References
-
- Clavin NW, Avraham T, Fernandez J, et al. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–2127. - PubMed
-
- Avraham T, Clavin NW, Daluvoy SV, et al. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg. 2009;124:438–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

