Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation
- PMID: 22456474
- PMCID: PMC4663993
- DOI: 10.1161/CIRCULATIONAHA.111.067306
Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation
Abstract
Background: Delayed afterdepolarizations (DADs) carried by Na(+)-Ca(2+)-exchange current (I(NCX)) in response to sarcoplasmic reticulum (SR) Ca(2+) leak can promote atrial fibrillation (AF). The mechanisms leading to delayed afterdepolarizations in AF patients have not been defined.
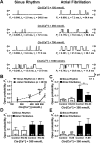
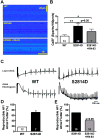
Methods and results: Protein levels (Western blot), membrane currents and action potentials (patch clamp), and [Ca(2+)](i) (Fluo-3) were measured in right atrial samples from 76 sinus rhythm (control) and 72 chronic AF (cAF) patients. Diastolic [Ca(2+)](i) and SR Ca(2+) content (integrated I(NCX) during caffeine-induced Ca(2+) transient) were unchanged, whereas diastolic SR Ca(2+) leak, estimated by blocking ryanodine receptors (RyR2) with tetracaine, was ≈50% higher in cAF versus control. Single-channel recordings from atrial RyR2 reconstituted into lipid bilayers revealed enhanced open probability in cAF samples, providing a molecular basis for increased SR Ca(2+) leak. Calmodulin expression (60%), Ca(2+)/calmodulin-dependent protein kinase-II (CaMKII) autophosphorylation at Thr287 (87%), and RyR2 phosphorylation at Ser2808 (protein kinase A/CaMKII site, 236%) and Ser2814 (CaMKII site, 77%) were increased in cAF. The selective CaMKII blocker KN-93 decreased SR Ca(2+) leak, the frequency of spontaneous Ca(2+) release events, and RyR2 open probability in cAF, whereas protein kinase A inhibition with H-89 was ineffective. Knock-in mice with constitutively phosphorylated RyR2 at Ser2814 showed a higher incidence of Ca(2+) sparks and increased susceptibility to pacing-induced AF compared with controls. The relationship between [Ca(2+)](i) and I(NCX) density revealed I(NCX) upregulation in cAF. Spontaneous Ca(2+) release events accompanied by inward I(NCX) currents and delayed afterdepolarizations/triggered activity occurred more often and the sensitivity of resting membrane voltage to elevated [Ca(2+)](i) (diastolic [Ca(2+)](i)-voltage coupling gain) was higher in cAF compared with control.
Conclusions: Enhanced SR Ca(2+) leak through CaMKII-hyperphosphorylated RyR2, in combination with larger I(NCX) for a given SR Ca(2+) release and increased diastolic [Ca(2+)](i)-voltage coupling gain, causes AF-promoting atrial delayed afterdepolarizations/triggered activity in cAF patients.
Conflict of interest statement
Conflict of Interest Disclosures
None.
Figures
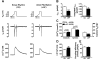
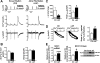


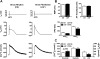
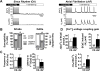
References
-
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. - PubMed
-
- Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol. 2008;52:293–299. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

