Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial
- PMID: 22456475
- PMCID: PMC3341507
- DOI: 10.1161/CIRCULATIONAHA.111.064113
Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial
Abstract
Background: Survivors of the Norwood procedure may experience neurodevelopmental impairment. Clinical trials to improve outcomes have focused primarily on methods of vital organ support during cardiopulmonary bypass.
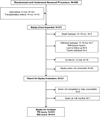
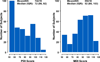
Methods and results: In the Single Ventricle Reconstruction trial of the Norwood procedure with modified Blalock-Taussig shunt versus right-ventricle-to-pulmonary-artery shunt, 14-month neurodevelopmental outcome was assessed by use of the Psychomotor Development Index (PDI) and Mental Development Index (MDI) of the Bayley Scales of Infant Development-II. We used multivariable regression to identify risk factors for adverse outcome. Among 373 transplant-free survivors, 321 (86%) returned at age 14.3 ± 1.1 (mean ± SD) months. Mean PDI (74 ± 19) and MDI (89 ± 18) scores were lower than normative means (each P<0.001). Neither PDI nor MDI score was associated with type of Norwood shunt. Independent predictors of lower PDI score (R(2)=26%) were clinical center (P=0.003), birth weight <2.5 kg (P=0.023), longer Norwood hospitalization (P<0.001), and more complications between Norwood procedure discharge and age 12 months (P<0.001). Independent risk factors for lower MDI score (R(2)=34%) included center (P<0.001), birth weight <2.5 kg (P=0.04), genetic syndrome/anomalies (P=0.04), lower maternal education (P=0.04), longer mechanical ventilation after the Norwood procedure (P<0.001), and more complications after Norwood discharge to age 12 months (P<0.001). We found no significant relationship of PDI or MDI score to perfusion type, other aspects of vital organ support (eg, hematocrit, pH strategy), or cardiac anatomy.
Conclusions: Neurodevelopmental impairment in Norwood survivors is more highly associated with innate patient factors and overall morbidity in the first year than with intraoperative management strategies. Improved outcomes are likely to require interventions that occur outside the operating room.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00115934.
Figures
References
-
- Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163–172. - PubMed
-
- Goldberg CS, Schwartz EM, Brunberg JA, et al. Neurodevelopmental outcome of patients after the Fontan operation: A comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137(5):646–652. - PubMed
-
- Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatr. 2000;105(5):1082–1089. - PubMed
-
- McCrindle BW, Williams RV, Mitchell PD, et al. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113(8):1123–1129. - PubMed
-
- Tabbutt S, Nord AS, Jarvik GP, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatr. 2008;121(3):476–483. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- HL068288/HL/NHLBI NIH HHS/United States
- K23 HD061601/HD/NICHD NIH HHS/United States
- HL068285/HL/NHLBI NIH HHS/United States
- U01 HL068279/HL/NHLBI NIH HHS/United States
- U01 HL068290/HL/NHLBI NIH HHS/United States
- U10 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068281/HL/NHLBI NIH HHS/United States
- UL1 TR000055/TR/NCATS NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- UL1 RR 025758/RR/NCRR NIH HHS/United States
- U01 HL085057/HL/NHLBI NIH HHS/United States
- HL068290/HL/NHLBI NIH HHS/United States
- U01 HL068269/HL/NHLBI NIH HHS/United States
- U10 HL109816/HL/NHLBI NIH HHS/United States
- HL068279/HL/NHLBI NIH HHS/United States
- U01 HL068288/HL/NHLBI NIH HHS/United States
- U01 HL068270/HL/NHLBI NIH HHS/United States
- HL085057/HL/NHLBI NIH HHS/United States
- HL068281/HL/NHLBI NIH HHS/United States
- U01 HL068292/HL/NHLBI NIH HHS/United States
- HL068269/HL/NHLBI NIH HHS/United States
- HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068285/HL/NHLBI NIH HHS/United States
- HL068292/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

