Redifferentiation of expanded human pancreatic β-cell-derived cells by inhibition of the NOTCH pathway
- PMID: 22457355
- PMCID: PMC3366837
- DOI: 10.1074/jbc.M111.319152
Redifferentiation of expanded human pancreatic β-cell-derived cells by inhibition of the NOTCH pathway
Abstract
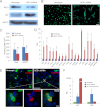
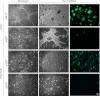
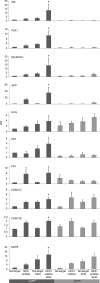
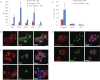
In vitro expansion of β-cells from adult human pancreatic islets would overcome donor β-cell shortage for cell replacement therapy for diabetes. Using a β-cell-specific labeling system we have shown that β-cell expansion is accompanied by dedifferentiation resembling epithelial-mesenchymal transition and loss of insulin expression. Epigenetic analyses indicate that key β-cell genes maintain open chromatin structure in expanded β-cell-derived (BCD) cells, although they are not transcribed. In the developing pancreas important cell-fate decisions are regulated by NOTCH receptors, which signal through the Hairy and Enhancer of Split 1 (HES1) transcription regulator. We have reported that BCD cell dedifferentiation and proliferation in vitro correlate with reactivation of the NOTCH pathway. Inhibition of HES1 expression using shRNA during culture initiation results in reduced β-cell replication and dedifferentiation, suggesting that HES1 inhibition may also affect BCD cell redifferentiation following expansion. Here, we used HES1 shRNA to down-regulate HES1 expression in expanded human BCD cells, showing that HES1 inhibition is sufficient to induce BCD cell redifferentiation, as manifested by a significant increase in insulin expression. Combined treatment with HES1 shRNA, cell aggregation in serum-free medium, and a mixture of soluble factors further stimulated the redifferentiation of BCD cells. In vivo analyses demonstrated the ability of the redifferentiated cells to replace β-cell function in hyperglycemic immunodeficient mice. These findings demonstrate the redifferentiation potential of ex vivo expanded BCD cells and the reproducible differentiating effect of HES1 inhibition in these cells.
Figures

References
-
- Meier J. J., Bhushan A., Butler A. E., Rizza R. A., Butler P. C. (2005) Sustained β cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48, 2221–2228 - PubMed
-
- Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 - PubMed
-
- Nielsen J. H., Brunstedt J., Andersson A., Frimodt-Møller C. (1979) Preservation of β cell function in adult human pancreatic islets for several months in vitro. Diabetologia 16, 97–100 - PubMed
-
- Hayek A., Beattie G. M., Cirulli V., Lopez A. D., Ricordi C., Rubin J. S. (1995) Growth factor/matrix-induced proliferation of human adult β-cells. Diabetes 44, 1458–1460 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

