Endothelin induces rapid, dynamin-mediated budding of endothelial caveolae rich in ET-B
- PMID: 22457360
- PMCID: PMC3366858
- DOI: 10.1074/jbc.M111.338897
Endothelin induces rapid, dynamin-mediated budding of endothelial caveolae rich in ET-B
Abstract
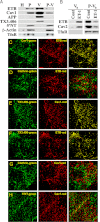
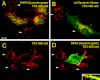
Clathrin-independent trafficking pathways for internalizing G protein-coupled receptors (GPCRs) remain undefined. Clathrin-mediated endocytosis of receptors including ligand-engaged GPCRs can be very rapid and comprehensive (<10 min). Caveolae-mediated endocytosis of ligands and antibodies has been reported to be much slower in cell culture (≫10 min). Little is known about the role of physiological ligands and specific GPCRs in regulating caveolae trafficking. Here, we find that one receptor for endothelin, ET-B but not ET-A, resides on endothelial cell surfaces in both tissue and cell culture primarily concentrated within caveolae. Reconstituted cell-free budding assays show that endothelins (ETs) induce the fission of caveolae from endothelial plasma membranes purified from rat lungs. Electron microcopy of lung tissue sections and tissue subcellular fractionation both show that endothelin administered intravascularly in rats also induces a significant loss of caveolae at the luminal surface of lung vascular endothelium. Endothelial cells in culture show that ET stimulates very rapid internalization of caveolae and cargo including caveolin, caveolae-targeting antibody, and itself. The ET-B inhibitor BQ788, but not the ET-A inhibitor BQ123, blocks the ET-induced budding of caveolae. Both the pharmacological inhibitor Dynasore and the genetic dominant negative K44A mutant of dynamin prevent this induced budding and internalization of caveolae. Also shRNA lentivirus knockdown of caveolin-1 expression prevents rapid internalization of ET and ET-B. It appears that endothelin can engage ET-B already highly concentrated in caveolae of endothelial cells to induce very rapid caveolae fission and endocytosis. This transport requires active dynamin function. Caveolae trafficking may occur more rapidly than previously documented when it is stimulated by a specific ligand to signaling receptors already located in caveolae before ligand engagement.
Figures





References
-
- Montesano R., Roth J., Robert A., Orci L. (1982) Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature 296, 651–653 - PubMed
-
- Schnitzer J. E., Oh P., McIntosh D. P. (1996) Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science 274, 239–242 - PubMed
-
- Pelkmans L., Kartenbeck J., Helenius A. (2001) Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473–483 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

