Photoreceptor coupling mediated by connexin36 in the primate retina
- PMID: 22457514
- PMCID: PMC3335500
- DOI: 10.1523/JNEUROSCI.4749-11.2012
Photoreceptor coupling mediated by connexin36 in the primate retina
Abstract
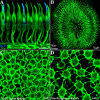
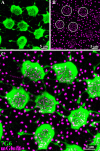
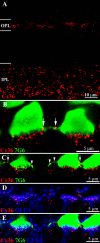
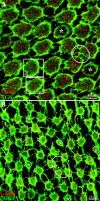
Photoreceptors are coupled via gap junctions in many mammalian species. Cone-to-cone coupling is thought to improve sensitivity and signal-to-noise ratio, while rod-to-cone coupling provides an alternative rod pathway active under twilight or mesopic conditions (Smith et al., 1986; DeVries et al., 2002; Hornstein et al., 2005). Gap junctions are composed of connexins, and connexin36 (Cx36), the dominant neuronal connexin, is expressed in the outer plexiform layer. Primate (Macaca mulatta) cone pedicles, labeled with an antibody against cone arrestin (7G6) were connected by a network of fine processes called telodendria and, in double-labeled material, Cx36 plaques were located precisely at telodendrial contacts between cones, suggesting strongly they are Cx36 gap junctions. Each red/green cone made nonselective connections with neighboring red/green cones. In contrast, blue cone pedicles were smaller with relatively few short telodendria and they made only rare or equivocal Cx36 contacts with adjacent cones. There were also many smaller Cx36 plaques around the periphery of every cone pedicle and along a series of very fine telodendria that were too short to reach adjacent members of the cone pedicle mosaic. These small Cx36 plaques were closely aligned with nearly every rod spherule and may identify sites of rod-to-cone coupling, even though the identity of the rod connexin has not been established. We conclude that the matrix of cone telodendria is the substrate for photoreceptor coupling. Red/green cones were coupled indiscriminately but blue cones were rarely connected with other cones. All cone types, including blue cones, made gap junctions with surrounding rod spherules.
Figures




References
-
- Ahnelt PK, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog Retin Eye Res. 2000;19:711–777. - PubMed
-
- Ahnelt PK, Kolb H, Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol. 1987;255:18–34. - PubMed
-
- Ahnelt P, Keri C, Kolb H. Identification of pedicles of putative blue-sensitive cones in the human retina. J Comp Neurol. 1990;293:39–53. - PubMed
-
- Attwell D, Borges S, Wu SM, Wilson M. Signal clipping by the rod output synapse. Nature. 1987;328:522–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
