The Epstein-Barr virus BcRF1 gene product is a TBP-like protein with an essential role in late gene expression
- PMID: 22457524
- PMCID: PMC3372218
- DOI: 10.1128/JVI.00159-12
The Epstein-Barr virus BcRF1 gene product is a TBP-like protein with an essential role in late gene expression
Abstract
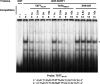
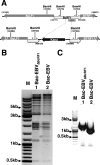
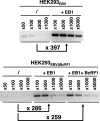
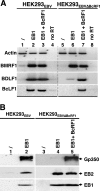
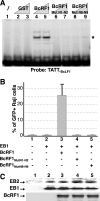
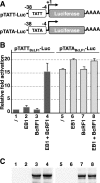
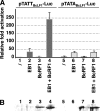
That the expression of late genes is coupled to viral genome replication is well established for all herpesviruses, but the exact mechanisms of their regulation, especially by viral proteins, are poorly understood. Here, we report the identification of the Epstein-Barr virus (EBV) early protein BcRF1 as a viral factor crucial for the activation of late gene transcription following viral DNA replication during the productive cycle. In order to study the function of the BcRF1 protein, we constructed a recombinant EBV lacking this gene. In HEK293 cells, this recombinant virus underwent normal DNA replication during the productive cycle but failed to express high levels of late gene transcripts or proteins, resulting in a nonproductive infection. Interestingly, a TATT motif is present in the promoter of most EBV late genes, at the position of the TATA box. We show here that BcRF1 forms a complex with the TATT motif and that this interaction is required for activation of late viral gene expression. Moreover, our results suggest that BcRF1 acts via interaction with other viral proteins.
Figures

References
-
- Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395–404 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

