Ultrastructural visualization of individual tegument protein dissociation during entry of herpes simplex virus 1 into human and rat dorsal root ganglion neurons
- PMID: 22457528
- PMCID: PMC3372220
- DOI: 10.1128/JVI.07016-11
Ultrastructural visualization of individual tegument protein dissociation during entry of herpes simplex virus 1 into human and rat dorsal root ganglion neurons
Abstract
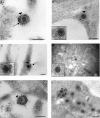
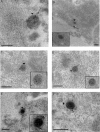
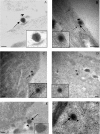
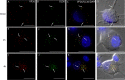
Herpes simplex virus 1 (HSV-1) enters neurons primarily by fusion of the viral envelope with the host cell plasma membrane, leading to the release of the capsid into the cytosol. The capsid travels via microtubule-mediated retrograde transport to the nuclear membrane, where the viral DNA is released for replication in the nucleus. In the present study, the composition and kinetics of incoming HSV-1 capsids during entry and retrograde transport in axons of human fetal and dissociated rat dorsal root ganglia (DRG) neurons were examined by wide-field deconvolution microscopy and transmission immunoelectron microscopy (TIEM). We show that HSV-1 tegument proteins, including VP16, VP22, most pUL37, and some pUL36, dissociated from the incoming virions. The inner tegument proteins, including pUL36 and some pUL37, remained associated with the capsid during virus entry and transit to the nucleus in the neuronal cell body. By TIEM, a progressive loss of tegument proteins, including VP16, VP22, most pUL37, and some pUL36, was observed, with most of the tegument dissociating at the plasma membrane of the axons and the neuronal cell body. Further dissociation occurred within the axons and the cytosol as the capsids moved to the nucleus, resulting in the release of free tegument proteins, especially VP16, VP22, pUL37, and some pUL36, into the cytosol. This study elucidates ultrastructurally the composition of HSV-1 capsids that encounter the microtubules in the core of human axons and the complement of free tegument proteins released into the cytosol during virus entry.
Figures


References
-
- Amici C, et al. 2006. Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J. Biol. Chem. 281:7110–7117 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

