Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation
- PMID: 22457593
- PMCID: PMC3310409
- DOI: 10.2147/IJN.S27159
Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation
Abstract
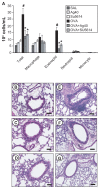
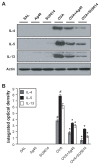
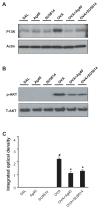
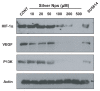
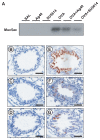
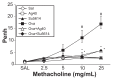
The anti-inflammatory action of silver nanoparticles (NPs) has been reported in a murine model of asthma in a previous study. But more specific mechanisms of silver NPs in an attenuation of allergic airway inflammation have not yet been established. Vascular and mucous changes are believed to contribute largely in pathophysiology in asthma. Among various factors related to vascular changes, vascular endothelial growth factor (VEGF) plays a pivotal role in vascular changes in asthma. Mucin proteins MUC5AC and MUC5B have been implicated as markers of goblet cell metaplasia in lung pathologies. The aim of this study was to investigate the effects of silver NPs on VEGF signaling pathways and mucus hypersecretion. Ovalbumin (OVA)-inhaled female BALBc mice were used to evaluate the role of silver NPs and the related molecular mechanisms in allergic airway disease. In this study, with an OVA-induced murine model of allergic airway disease, it was found that the increased levels of hypoxia-inducible factor (HIF)-1α, VEGF, phosphatidylinositol-3 kinase (PI3K) and phosphorylated-Akt levels, and mucous glycoprotein expression (Muc5ac) in lung tissues were substantially decreased by the administration of silver NPs. In summary, silver NPs substantially suppressed mucus hypersecretion and PI3K/HIF-1α/VEGF signaling pathway in an allergic airway inflammation.
Keywords: allergic airway disease; hypoxia inducible factor-1α; vascular endothelial growth factor.
Figures



References
-
- Holgate ST, Lemanske RF, Jr, O’Byrne PM, Kakumanu S, Busse WW. Asthma pathogenesis. In: Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FE, editors. Middleton’s Allergy Principles and Practice. 7th ed. Vol. 2. Philadelphia, PA: Mosby Elsevier Inc; 2009. p. 911.
-
- Lee YC, Lee HK. Vascular endothelial growth factor in patients with acute asthma. J Allergy Clin Immunol. 2001;107:1106–1108. - PubMed
-
- McCullagh A, Rosenthal M, Wanner A, et al. The bronchial circulation-worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatr Pulmonol. 2010;45:1–13. - PubMed
-
- Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

