Dengue virus infection perturbs lipid homeostasis in infected mosquito cells
- PMID: 22457619
- PMCID: PMC3310792
- DOI: 10.1371/journal.ppat.1002584
Dengue virus infection perturbs lipid homeostasis in infected mosquito cells
Abstract
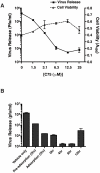
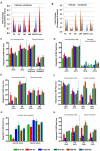
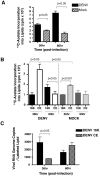
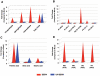
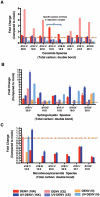
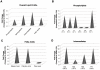
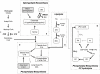
Dengue virus causes ∼50-100 million infections per year and thus is considered one of the most aggressive arthropod-borne human pathogen worldwide. During its replication, dengue virus induces dramatic alterations in the intracellular membranes of infected cells. This phenomenon is observed both in human and vector-derived cells. Using high-resolution mass spectrometry of mosquito cells, we show that this membrane remodeling is directly linked to a unique lipid repertoire induced by dengue virus infection. Specifically, 15% of the metabolites detected were significantly different between DENV infected and uninfected cells while 85% of the metabolites detected were significantly different in isolated replication complex membranes. Furthermore, we demonstrate that intracellular lipid redistribution induced by the inhibition of fatty acid synthase, the rate-limiting enzyme in lipid biosynthesis, is sufficient for cell survival but is inhibitory to dengue virus replication. Lipids that have the capacity to destabilize and change the curvature of membranes as well as lipids that change the permeability of membranes are enriched in dengue virus infected cells. Several sphingolipids and other bioactive signaling molecules that are involved in controlling membrane fusion, fission, and trafficking as well as molecules that influence cytoskeletal reorganization are also up regulated during dengue infection. These observations shed light on the emerging role of lipids in shaping the membrane and protein environments during viral infections and suggest membrane-organizing principles that may influence virus-induced intracellular membrane architecture.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

