The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization
- PMID: 22457634
- PMCID: PMC3310732
- DOI: 10.1371/journal.pgen.1002561
The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization
Abstract
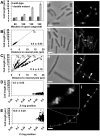
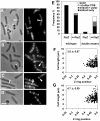
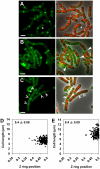
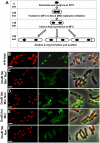
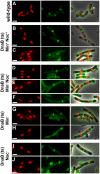
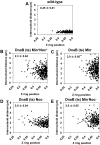
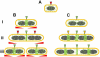
Precise temporal and spatial control of cell division is essential for progeny survival. The current general view is that precise positioning of the division site at midcell in rod-shaped bacteria is a result of the combined action of the Min system and nucleoid (chromosome) occlusion. Both systems prevent assembly of the cytokinetic Z ring at inappropriate places in the cell, restricting Z rings to the correct site at midcell. Here we show that in the bacterium Bacillus subtilis Z rings are positioned precisely at midcell in the complete absence of both these systems, revealing the existence of a mechanism independent of Min and nucleoid occlusion that identifies midcell in this organism. We further show that Z ring assembly at midcell is delayed in the absence of Min and Noc proteins, while at the same time FtsZ accumulates at other potential division sites. This suggests that a major role for Min and Noc is to ensure efficient utilization of the midcell division site by preventing Z ring assembly at potential division sites, including the cell poles. Our data lead us to propose a model in which spatial regulation of division in B. subtilis involves identification of the division site at midcell that requires Min and nucleoid occlusion to ensure efficient Z ring assembly there and only there, at the right time in the cell cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Harry E, Monahan L, Thompson L. Bacterial cell division: the mechanism and its precison. Int Rev Cytol. 2006;253:27–94. - PubMed
-
- Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat Rev Microbiol. 2005;3:959–968. - PubMed
-
- Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. - PubMed
-
- Mingorance J, Rivas G, Velez M, Gomez-Puertas P, Vicente M. Strong FtsZ is with the force: mechanisms to constrict bacteria. Trends Microbiol. 2010;18:348–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

