Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages
- PMID: 22457638
- PMCID: PMC3310790
- DOI: 10.1371/journal.pgen.1002592
Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages
Abstract
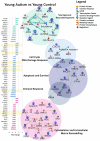
Autism is a highly heritable neurodevelopmental disorder, yet the genetic underpinnings of the disorder are largely unknown. Aberrant brain overgrowth is a well-replicated observation in the autism literature; but association, linkage, and expression studies have not identified genetic factors that explain this trajectory. Few studies have had sufficient statistical power to investigate whole-genome gene expression and genotypic variation in the autistic brain, especially in regions that display the greatest growth abnormality. Previous functional genomic studies have identified possible alterations in transcript levels of genes related to neurodevelopment and immune function. Thus, there is a need for genetic studies involving key brain regions to replicate these findings and solidify the role of particular functional pathways in autism pathogenesis. We therefore sought to identify abnormal brain gene expression patterns via whole-genome analysis of mRNA levels and copy number variations (CNVs) in autistic and control postmortem brain samples. We focused on prefrontal cortex tissue where excess neuron numbers and cortical overgrowth are pronounced in the majority of autism cases. We found evidence for dysregulation in pathways governing cell number, cortical patterning, and differentiation in young autistic prefrontal cortex. In contrast, adult autistic prefrontal cortex showed dysregulation of signaling and repair pathways. Genes regulating cell cycle also exhibited autism-specific CNVs in DNA derived from prefrontal cortex, and these genes were significantly associated with autism in genome-wide association study datasets. Our results suggest that CNVs and age-dependent gene expression changes in autism may reflect distinct pathological processes in the developing versus the mature autistic prefrontal cortex. Our results raise the hypothesis that genetic dysregulation in the developing brain leads to abnormal regional patterning, excess prefrontal neurons, cortical overgrowth, and neural dysfunction in autism.
Conflict of interest statement
J-BF and CA declare stock and employment interest in Illumina, Inc.
Figures



References
-
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. - PubMed
-
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. NeuroImage. 2002;16:1038–1051. - PubMed
-
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH078151/MH/NIMH NIH HHS/United States
- R01 AG031224/AG/NIA NIH HHS/United States
- U19 AG023122/AG/NIA NIH HHS/United States
- P50-MH081755/MH/NIMH NIH HHS/United States
- U01 DA024417/DA/NIDA NIH HHS/United States
- U19 AG023122-01/AG/NIA NIH HHS/United States
- U54 NS056883/NS/NINDS NIH HHS/United States
- U01 DA024417-01/DA/NIDA NIH HHS/United States
- AS2111/Autism Speaks/United States
- P50 MH081755/MH/NIMH NIH HHS/United States
- N01 MH022005/MH/NIMH NIH HHS/United States
- RC2 DA029475/DA/NIDA NIH HHS/United States
- R01 MH078151-01A1/MH/NIMH NIH HHS/United States
- UL1 RR025774/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

