Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice
- PMID: 22457829
- PMCID: PMC3311621
- DOI: 10.1371/journal.pone.0034233
Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice
Abstract
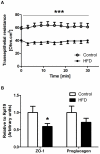
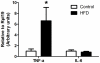
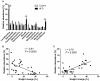
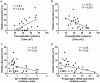
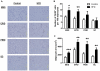
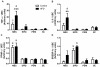
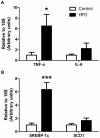
We investigated the relationship between gut health, visceral fat dysfunction and metabolic disorders in diet-induced obesity. C57BL/6J mice were fed control or high saturated fat diet (HFD). Circulating glucose, insulin and inflammatory markers were measured. Proximal colon barrier function was assessed by measuring transepithelial resistance and mRNA expression of tight-junction proteins. Gut microbiota profile was determined by 16S rDNA pyrosequencing. Tumor necrosis factor (TNF)-α and interleukin (IL)-6 mRNA levels were measured in proximal colon, adipose tissue and liver using RT-qPCR. Adipose macrophage infiltration (F4/80⁺) was assessed using immunohistochemical staining. HFD mice had a higher insulin/glucose ratio (P = 0.020) and serum levels of serum amyloid A3 (131%; P = 0.008) but reduced circulating adiponectin (64%; P = 0.011). In proximal colon of HFD mice compared to mice fed the control diet, transepithelial resistance and mRNA expression of zona occludens 1 were reduced by 38% (P<0.001) and 40% (P = 0.025) respectively and TNF-α mRNA level was 6.6-fold higher (P = 0.037). HFD reduced Lactobacillus (75%; P<0.001) but increased Oscillibacter (279%; P = 0.004) in fecal microbiota. Correlations were found between abundances of Lactobacillus (r = 0.52; P = 0.013) and Oscillibacter (r = -0.55; P = 0.007) with transepithelial resistance of the proximal colon. HFD increased macrophage infiltration (58%; P = 0.020), TNF-α (2.5-fold, P<0.001) and IL-6 mRNA levels (2.5-fold; P = 0.008) in mesenteric fat. Increased macrophage infiltration in epididymal fat was also observed with HFD feeding (71%; P = 0.006) but neither TNF-α nor IL-6 was altered. Perirenal and subcutaneous adipose tissue showed no signs of inflammation in HFD mice. The current results implicate gut dysfunction, and attendant inflammation of contiguous adipose, as salient features of the metabolic dysregulation of diet-induced obesity.
Conflict of interest statement
Figures

References
-
- Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, et al. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283:E1135–1143. - PubMed
-
- Barbarroja N, Lopez-Pedrera R, Mayas MD, Garcia-Fuentes E, Garrido-Sanchez L, et al. The obese healthy paradox: is inflammation the answer? Biochem J. 2010;430:141–149. - PubMed
-
- Lam YY, Janovska A, McAinch AJ, Belobrajdic DP, Hatzinikolas G, et al. The use of adipose tissue-conditioned media to demonstrate the differential effects of fat depots on insulin-stimulated glucose uptake in a skeletal muscle cell line. Obes Res Clin Pract. 2011;5:e43–e54. - PubMed
-
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

