Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management
- PMID: 22457972
- PMCID: PMC5373096
- DOI: 10.1146/annurev-marine-120308-081121
Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management
Abstract
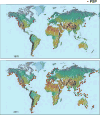
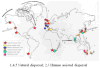
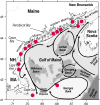
The public health, tourism, fisheries, and ecosystem impacts from harmful algal blooms (HABs) have all increased over the past few decades. This has led to heightened scientific and regulatory attention, and the development of many new technologies and approaches for research and management. This, in turn, is leading to significant paradigm shifts with regard to, e.g., our interpretation of the phytoplankton species concept (strain variation), the dogma of their apparent cosmopolitanism, the role of bacteria and zooplankton grazing in HABs, and our approaches to investigating the ecological and genetic basis for the production of toxins and allelochemicals. Increasingly, eutrophication and climate change are viewed and managed as multifactorial environmental stressors that will further challenge managers of coastal resources and those responsible for protecting human health. Here we review HAB science with an eye toward new concepts and approaches, emphasizing, where possible, the unexpected yet promising new directions that research has taken in this diverse field.
Figures






References
-
- Adolf JE, Place AR, Stoecker DK, Harding LV. Modulation of polyunsaturated fatty acids in mixotrophic Karlodinium veneficum (Dinophyceae) and its prey, Storeatula major (Cryptophyceae)l. J Phycol. 2007;43(6):1259–70.
-
- Alpermann TJ, Beszteri B, John U, Tillmann U, Cembella AD. Implications of life-history transitions on the population genetic structure of the toxigenic marine dinoflagellate Alexandrium tamarense. Mol Ecol. 2009;18(10):2122–33. - PubMed
-
- Alpermann TJ, Tillmann U, Beszteri B, Cembella AD, John U. Phenotypic variation and genotypic diversity in a planktonic population of the toxigenic marine dinoflagellate Alexandrium tamarense (Dinophyceae) J Phycol. 2010;46(1):18–32.
-
- Andersen RA, editor. Algal culturing techniques. Elsevier; NY: 2005. p. 596.
-
- Anderson DM. Toxic algal blooms and red tides: a global perspective. In: Okaichi T, Anderson DM, Nemoto T, editors. Red Tides: Biology, Environmental Science and Toxicology. Elsevier; 1989. pp. 11–16.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

