Friedel-Crafts amidoalkylation via thermolysis and oxidative photocatalysis
- PMID: 22458307
- PMCID: PMC3360956
- DOI: 10.1021/jo300162c
Friedel-Crafts amidoalkylation via thermolysis and oxidative photocatalysis
Abstract
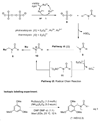
Friedel-Crafts amidoalkylation was achieved by oxidation of dialkylamides using persulfate (S(2)O(8)(2-)) in the presence of the visible light catalyst, Ru(bpy)(3)Cl(2), at room temperature, via a reactive N-acyliminium intermediate. Alternatively, mild heating of the dialkylamides and persulfate afforded a metal and Lewis acid-free Friedel-Crafts amidoalkylation. Alcohols and electron-rich arenes served as effective nucleophiles, forming new C-O or C-C bonds. In general, photocatalysis provided higher yields and better selectivities.
Figures
References
-
-
For recent reviews on photoredox catalysis, see: Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102–113. Teplý F. Collect. Czech. Chem. Commun. 2011;76:859–917. Tucker JW, Stephenson CRJ. J. Org. Chem. 2012;77:1617–1622.
-
-
-
For selected recent examples of photoredox catalysis in organic synthesis, see: Nicewicz DA, MacMillan DWC. Science. 2008;322:77–80. Ischay MA, Anzovino ME, Du J, Yoon TP. J. Am. Chem. Soc. 2008;130:12886–12887. Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756–8757. Shih H-W, Vander Wal MN, Grange RL, MacMillan DWC. J. Am. Chem. Soc. 2010;132:13600–13603. Andrews SR, Becker JJ, Gagne MR. Angew. Chem. Int. Ed. 2010;49:7274–7276. Ischay MA, Lu Z, Yoon TP. J. Am. Chem. Soc. 2010;132:8572–8574. Neumann M, Füldner S, König B, Zeitler K. Angew. Chem. Int. Ed. 2011;50:951–954. Lu Z, Shen M, Yoon TP. J. Am. Chem. Soc. 2011;133:1162–1164. C. Dai C, Narayanam JMR, Stephenson CRJ. Nature Chem. 2011;3:140–145. Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J. Am. Chem. Soc. 2011;133:4160–4163. Furst L, Narayanam JMR, Stephenson CRJ. Angew. Chem. Int. Ed. 2011;50:9655–9659.
-
-
-
Freeman DB, Furst L, Condie AG, Stephenson CRJ. Org. Lett. 2012;14:94–97. (b) For a previous study using Ir(ppy)2(dtbbpy)PF6 as a catalyst, see: Condie AG, González-Gómez JC, Stephenson CRJ. J. Am. Chem. Soc. 2010;132:1464–1465.
-
-
-
For selected recent examples of the oxidative functionalization of tertiary amines using photoredox catalysis, see: Rueping M, Vila C, Koenigs RM, Poscharny K, Fabry DC. Chem. Commun. 2011;47:2360–2362. Hari DP, König B. Org. Lett. 2011;13:3852–3855. Zou Y-Q, Lu L-Q, Fu L, Chang N-J, Rong J, Chen J-R, Xiao W-J. Angew. Chem. Int. Ed. 2011;50:7171–7175.
-
-
-
For selected recent examples of the applications of N-acyliminium ions, see: Sun H, Martin C, Kesselring D, Keller R, Moeller KD. J. Am. Chem. Soc. 2006;128:13761–13771. Maruyama T, Mizuno Y, Shimizu I, Suga S, Yoshida J-I. J. Am. Chem. Soc. 2007;129:1902–1903. Pilling AW, Boehmer J, Dixon DJ. Angew. Chem. Int. Ed. 2007;46:5428–5430. Othman RB, Affani R, Tranchant M-J, Antoniotti S, Dalla V, Duñach E. Angew. Chem. Int. Ed. 2010;49:776–780.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources