Tailored enrichment strategy detects low abundant small noncoding RNAs in HIV-1 infected cells
- PMID: 22458358
- PMCID: PMC3341194
- DOI: 10.1186/1742-4690-9-27
Tailored enrichment strategy detects low abundant small noncoding RNAs in HIV-1 infected cells
Abstract
Background: The various classes of small noncoding RNAs (sncRNAs) are important regulators of gene expression across divergent types of organisms. While a rapidly increasing number of sncRNAs has been identified over recent years, the isolation of sncRNAs of low abundance remains challenging. Virally encoded sncRNAs, particularly those of RNA viruses, can be expressed at very low levels. This is best illustrated by HIV-1 where virus encoded sncRNAs represent approximately 0.1-1.0% of all sncRNAs in HIV-1 infected cells or were found to be undetected. Thus, we applied a novel, sequence targeted enrichment strategy to capture HIV-1 derived sncRNAs in HIV-1 infected primary CD4+ T-lymphocytes and macrophages that allows a greater than 100-fold enrichment of low abundant sncRNAs.
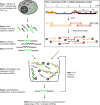
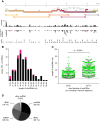
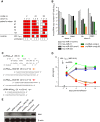
Results: Eight hundred and ninety-two individual HIV-1 sncRNAs were cloned and sequenced from nine different sncRNA libraries derived from five independent experiments. These clones represent up to 90% of all sncRNA clones in the generated libraries. Two hundred and sixteen HIV-1 sncRNAs were distinguishable as unique clones. They are spread throughout the HIV-1 genome, however, forming certain clusters, and almost 10% show an antisense orientation. The length of HIV-1 sncRNAs varies between 16 and 89 nucleotides with an unexpected peak at 31 to 50 nucleotides, thus, longer than cellular microRNAs or short-interfering RNAs (siRNAs). Exemplary HIV-1 sncRNAs were also generated in cells infected with different primary HIV-1 isolates and can inhibit HIV-1 replication.
Conclusions: HIV-1 infected cells generate virally encoded sncRNAs, which might play a role in the HIV-1 life cycle. Furthermore, the enormous capacity to enrich low abundance sncRNAs in a sequence specific manner highly recommends our selection strategy for any type of investigation where origin or target sequences of the sought-after sncRNAs are known.
Figures

References
-
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

