The generation of vertebral segmental patterning in the chick embryo
- PMID: 22458512
- PMCID: PMC3390512
- DOI: 10.1111/j.1469-7580.2012.01497.x
The generation of vertebral segmental patterning in the chick embryo
Abstract
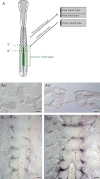
We have carried out a series of experimental manipulations in the chick embryo to assess whether the notochord, neural tube and spinal nerves influence segmental patterning of the vertebral column. Using Pax1 expression in the somite-derived sclerotomes as a marker for segmentation of the developing intervertebral disc, our results exclude such an influence. In contrast to certain teleost species, where the notochord has been shown to generate segmentation of the vertebral bodies (chordacentra), these experiments indicate that segmental patterning of the avian vertebral column arises autonomously in the somite mesoderm. We suggest that in amniotes, the subdivision of each sclerotome into non-miscible anterior and posterior halves plays a critical role in establishing vertebral segmentation, and in maintaining left/right alignment of the developing vertebral elements at the body midline.
© 2012 The Authors. Journal of Anatomy © 2012 Anatomical Society.
Figures




Similar articles
-
The role of the notochord in amniote vertebral column segmentation.Dev Biol. 2018 Jul 1;439(1):3-18. doi: 10.1016/j.ydbio.2018.04.005. Epub 2018 Apr 11. Dev Biol. 2018. PMID: 29654746 Free PMC article.
-
Patterning spinal nerves and vertebral bones.J Anat. 2018 Apr;232(4):534-539. doi: 10.1111/joa.12714. Epub 2017 Oct 24. J Anat. 2018. PMID: 29063597 Free PMC article. Review.
-
A central role for the notochord in vertebral patterning.Development. 2004 Feb;131(4):873-80. doi: 10.1242/dev.00952. Epub 2004 Jan 21. Development. 2004. PMID: 14736741
-
Notochord-dependent expression of MFH1 and PAX1 cooperates to maintain the proliferation of sclerotome cells during the vertebral column development.Dev Biol. 1999 Jun 1;210(1):15-29. doi: 10.1006/dbio.1999.9261. Dev Biol. 1999. PMID: 10364424
-
Formation and differentiation of the avian sclerotome.Anat Embryol (Berl). 2004 Aug;208(5):333-50. doi: 10.1007/s00429-004-0408-z. Epub 2004 Jul 28. Anat Embryol (Berl). 2004. PMID: 15309628 Review.
Cited by
-
A resegmentation-shift model for vertebral patterning.J Anat. 2017 Feb;230(2):290-296. doi: 10.1111/joa.12540. Epub 2016 Sep 1. J Anat. 2017. PMID: 27580767 Free PMC article.
-
The role of the notochord in amniote vertebral column segmentation.Dev Biol. 2018 Jul 1;439(1):3-18. doi: 10.1016/j.ydbio.2018.04.005. Epub 2018 Apr 11. Dev Biol. 2018. PMID: 29654746 Free PMC article.
-
Mineralization of the vertebral bodies in Atlantic salmon (Salmo salar L.) is initiated segmentally in the form of hydroxyapatite crystal accretions in the notochord sheath.J Anat. 2013 Aug;223(2):159-70. doi: 10.1111/joa.12067. Epub 2013 May 27. J Anat. 2013. PMID: 23711083 Free PMC article.
-
Patterning spinal nerves and vertebral bones.J Anat. 2018 Apr;232(4):534-539. doi: 10.1111/joa.12714. Epub 2017 Oct 24. J Anat. 2018. PMID: 29063597 Free PMC article. Review.
-
Transcriptome sequencing of Atlantic salmon (Salmo salar L.) notochord prior to development of the vertebrae provides clues to regulation of positional fate, chordoblast lineage and mineralisation.BMC Genomics. 2014 Feb 19;15:141. doi: 10.1186/1471-2164-15-141. BMC Genomics. 2014. PMID: 24548379 Free PMC article.
References
-
- Aoyama H, Asamoto K. The developmental fate of the rostral/caudal half of a somite for vertebra and rib formation: experimental confirmation of the resegmentation theory using chick-quail chimeras. Mech Dev. 2000;99:71–82. - PubMed
-
- Bagnall KM, Sanders EJ. The binding pattern of peanut lectin associated with sclerotome migration and the formation of the vertebral axis in the chick embryo. Anat Embryol (Berl) 1989;180:505–513. - PubMed
-
- Bagnall KM, Higgins SJ, Sanders EJ. The contribution made by a single somite to the vertebral column: experimental evidence in support of resegmentation using the chick-quail chimaera model. Development. 1988;103:69–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

