Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition
- PMID: 22458763
- PMCID: PMC4898201
- DOI: 10.1042/BJ20120294
Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition
Abstract
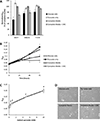
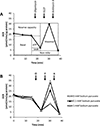
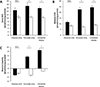
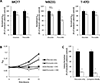
Recent studies have highlighted the fact that cancer cells have an altered metabolic phenotype, and this metabolic reprogramming is required to drive the biosynthesis pathways necessary for rapid replication and proliferation. Specifically, the importance of citric acid cycle-generated intermediates in the regulation of cancer cell proliferation has been recently appreciated. One function of MCTs (monocarboxylate transporters) is to transport the citric acid cycle substrate pyruvate across the plasma membrane and into mitochondria, and inhibition of MCTs has been proposed as a therapeutic strategy to target metabolic pathways in cancer. In the present paper, we examined the effect of different metabolic substrates (glucose and pyruvate) on mitochondrial function and proliferation in breast cancer cells. We demonstrated that cancer cells proliferate more rapidly in the presence of exogenous pyruvate when compared with lactate. Pyruvate supplementation fuelled mitochondrial oxygen consumption and the reserve respiratory capacity, and this increase in mitochondrial function correlated with proliferative potential. In addition, inhibition of cellular pyruvate uptake using the MCT inhibitor α-cyano-4-hydroxycinnamic acid impaired mitochondrial respiration and decreased cell growth. These data demonstrate the importance of mitochondrial metabolism in proliferative responses and highlight a novel mechanism of action for MCT inhibitors through suppression of pyruvate-fuelled mitochondrial respiration.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem.Physiol B Biochem.Mol.Biol. 1998;120:89–107. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat.Rev.Cancer. 2011;11:85–95. - PubMed
-
- Chen H, Wang L, Beretov J, Hao J, Xiao W, Li Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin.Exp.Metastasis. 2010;27:557–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

