Mitochondrial Ca(2+) signals in autophagy
- PMID: 22459281
- PMCID: PMC3389293
- DOI: 10.1016/j.ceca.2012.03.001
Mitochondrial Ca(2+) signals in autophagy
Abstract
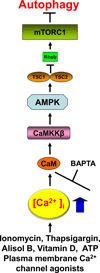
Macroautophagy (autophagy) is a lysosomal degradation pathway that is conserved from yeast to humans that plays an important role in recycling cellular constituents in all cells. A number of protein complexes and signaling pathways impinge on the regulation of autophagy, with the mammalian target of rapamycin (mTOR) as the central player in the canonical pathway. Cytoplasmic Ca(2+) signaling also regulates autophagy, with both activating and inhibitory effects, mediated by the canonical as well as non-canonical pathways. Here we review this regulation, with a focus on the role of an mTOR-independent pathway that involves the inositol trisphosphate receptor (InsP(3)R) Ca(2+) release channel and Ca(2+) signaling to mitochondria. Constitutive InsP(3)R Ca(2+) transfer to mitochondria is required for autophagy suppression in cells in nutrient-replete media. In its absence, cells become metabolically compromised due to insufficient production of reducing equivalents to support oxidative phosphorylation. Absence of this Ca(2+) transfer to mitochondria results in activation of AMPK, which activates mTOR-independent pro-survival autophagy. Constitutive InsP(3)R Ca(2+) release to mitochondria is an essential cellular process that is required for efficient mitochondrial respiration, maintenance of normal cell bioenergetics and suppression of autophagy.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. - PubMed
-
- Decuypere JP, Bultynck G, Parys JB. A dual role for Ca2+ in autophagy regulation. Cell Calcium. 2011;50:242–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

